Press release
Clinical Trial Management System (CTMS) Market: Growth, Opportunities, and Challenges
IntroductionThe Clinical Trial Management System (CTMS) market is a crucial segment of the global healthcare and pharmaceutical industries, which supports the efficient and accurate management of clinical trials. CTMS refers to software systems used by pharmaceutical, biotechnology, and contract research organizations (CROs) to streamline the planning, tracking, and management of clinical trials. These systems provide centralized data management for clinical trial activities, which helps improve the speed, quality, and compliance of trials. As clinical trials become increasingly complex, the need for robust CTMS solutions grows, prompting continuous innovation and evolution in the market.
For more information:
https://www.databridgemarketresearch.com/reports/global-clinical-trial-management-system-market
Market Size
Data Bridge Market Research analyses that the global clinical trial management system (CTMS) market size was valued at USD 1.25 billion in 2023, is projected to reach USD 3.41 billion by 2031, with a CAGR of 13.40% during the forecast period 2024 to 2031. This indicates that the market value. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Market Share
The CTMS market is dominated by a few key players who offer advanced solutions to healthcare organizations. These include large software providers such as Medidata Solutions, Veeva Systems, Parexel, Oracle, and IBM. Among these, Medidata Solutions holds a substantial share of the market due to its comprehensive suite of products that support end-to-end clinical trial management. Veeva Systems also maintains a significant market presence, particularly with its cloud-based offerings that cater to both large enterprises and small to medium-sized clinical research organizations.
North America accounts for the largest share of the global CTMS market, followed by Europe. The U.S. is a major contributor, with high adoption of advanced clinical trial technologies and a large number of pharmaceutical companies and clinical research organizations. Asia-Pacific, particularly China and India, is expected to witness rapid growth in the coming years, driven by an increase in clinical trial activities, rising healthcare infrastructure, and greater government support for pharmaceutical development.
Market Opportunities and Challenges
The CTMS market is rife with opportunities, but it also faces several challenges. On the opportunities front, the growing focus on personalized medicine and the increasing number of clinical trials being conducted globally are expected to drive the demand for CTMS solutions. Personalized medicine requires more targeted and smaller patient cohorts, often leading to more intricate trial management needs. The ability of CTMS software to manage diverse data sources, streamline patient recruitment, and improve regulatory compliance makes it an essential tool for such trials.
Moreover, the integration of artificial intelligence (AI) and machine learning (ML) with CTMS solutions presents another significant opportunity. These technologies can automate routine tasks, enhance data analytics, and provide predictive insights, thus further improving the efficiency and accuracy of clinical trials. As pharmaceutical companies increasingly adopt digital technologies to reduce operational costs, the demand for AI-enhanced CTMS systems is expected to rise.
However, the CTMS market also faces challenges. One of the primary hurdles is the high cost of implementation, which can be a barrier for smaller pharmaceutical companies or contract research organizations with limited budgets. These organizations may find it difficult to justify the investment required for implementing sophisticated CTMS solutions, especially when their clinical trial management processes are still largely paper-based or reliant on legacy systems. Additionally, integrating new CTMS solutions with existing systems can be complex and time-consuming, adding to the overall cost.
Another challenge is the increasing regulatory pressure on clinical trials, especially in regions with stringent requirements such as the European Union and the U.S. The need for CTMS systems to comply with various regulations, such as 21 CFR Part 11 in the U.S. or the European Medicines Agency (EMA) guidelines, means that software providers must constantly update their systems to meet changing requirements. Failure to comply with these regulations can result in delays, penalties, or even the invalidation of trial data, making regulatory compliance a critical concern for CTMS users.
Market Demand
The demand for CTMS solutions is primarily driven by the rising number of clinical trials, the complexity of modern trials, and the increasing regulatory requirements. As pharmaceutical and biotechnology companies develop new drugs and therapies, the need for effective trial management grows. The growing trend of outsourcing clinical trials to contract research organizations (CROs) is also fueling the demand for CTMS. CROs rely on these systems to handle multiple trials simultaneously, ensuring that data is accurate, secure, and compliant with international standards.
The shift toward decentralized clinical trials (DCTs) is another factor contributing to market demand. DCTs, which rely on remote patient monitoring and virtual trial sites, require robust CTMS solutions capable of integrating data from various sources, including telemedicine platforms, mobile health devices, and electronic health records (EHR). The demand for CTMS systems that can accommodate these new technologies and provide real-time data analysis is expected to rise significantly in the coming years.
Additionally, as the healthcare landscape becomes more data-driven, the ability to manage vast amounts of data from clinical trials is a growing concern. CTMS solutions that offer data analytics capabilities and real-time reporting features are in high demand. These solutions not only help in managing day-to-day trial operations but also assist in analyzing the data to draw insights that can accelerate drug development.
Market Trends
The CTMS market is evolving rapidly, with several key trends shaping its future. One prominent trend is the increasing shift toward cloud-based CTMS solutions. Cloud computing offers numerous advantages, including cost-effectiveness, scalability, and the ability to access trial data from anywhere in the world. This is particularly important in today's globalized pharmaceutical industry, where trials are often conducted across multiple countries and regions. Cloud-based CTMS platforms also enable real-time collaboration between stakeholders, such as sponsors, clinical investigators, and regulatory bodies, making it easier to manage complex trial logistics.
Another key trend is the growing focus on integration with other technologies, such as electronic data capture (EDC) systems, clinical data management systems (CDMS), and pharmacovigilance software. Integration between these platforms ensures seamless data flow and reduces the risk of errors or data inconsistencies. For example, CTMS systems integrated with EDC can automate data entry, reducing manual work and improving data accuracy. Similarly, integration with CDMS can streamline the management of clinical trial data and ensure its compliance with regulatory standards.
Moreover, there is an increasing emphasis on patient-centric clinical trials. Companies are adopting patient engagement tools and technologies that help improve recruitment and retention rates. CTMS providers are responding to this trend by developing solutions that incorporate patient engagement features such as mobile apps, patient portals, and digital consent forms. These tools improve patient experience and ensure better compliance with trial protocols.
Browse Trending Reports:
https://newsresearch12.blogspot.com/2024/11/high-temperature-insulation-materials_18.html
https://newsresearch12.blogspot.com/2024/11/anesthesia-dolorosa-treatment-market_18.html
https://newsresearch12.blogspot.com/2024/11/telemental-health-market-size-share.html
https://newsresearch12.blogspot.com/2024/11/capnography-equipment-market-size-share.html
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: corporatesales@databridgemarketresearch.com"
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Management System (CTMS) Market: Growth, Opportunities, and Challenges here
News-ID: 3743395 • Views: …
More Releases from Data Bridge Market Research

Scented Candle Market Shows Strong Growth Driven by Wellness and Home Décor Tr …
The global scented candle market is on track for significant expansion, increasing from an estimated USD 3.60 billion in 2024 to USD 6.00 billion by 2032, registering a strong CAGR of 6.60%. Rising consumer interest in home ambiance, wellness, and premium lifestyle products continues to drive market demand.
Get More Detail: https://www.databridgemarketresearch.com/reports/global-scented-candle-market
Market Growth Drivers
The scented candle market has evolved beyond being just a decorative item. Key growth factors include:
Home Fragrance &…
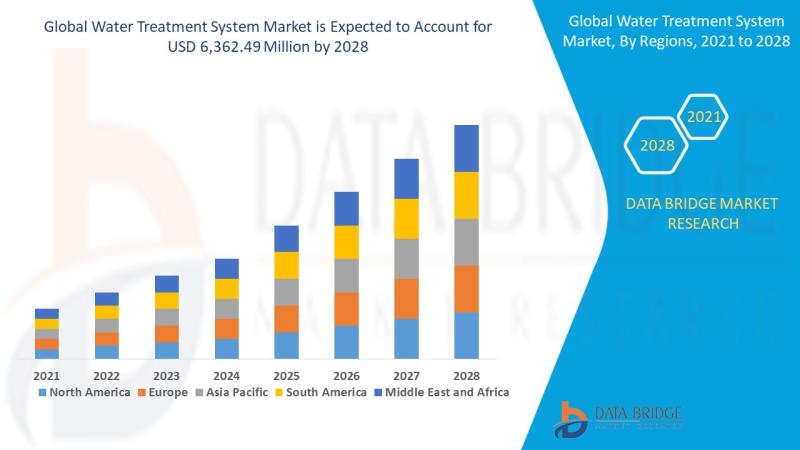
Water Treatment System Market: Sustaining the Future of Clean Water
Introduction
Understanding Water Treatment Systems
Water treatment systems are designed to purify and disinfect water for various uses-drinking, industrial processes, irrigation, and wastewater reuse. These systems eliminate contaminants such as bacteria, viruses, heavy metals, chemicals, and particulates, making water safe and sustainable for consumption and use.
Importance in Global Sustainability
Clean water is essential to life and industrial progress. With growing water demand and pollution, water treatment systems are now critical infrastructure across the…

Veterinary X-Ray Market Size, Analysis, Scope, Demand, Opportunities, Statistics
According to Data Bridge Market Research The global Veterinary X-Ray market size was valued at USD 915.19 million in 2024 and is projected to reach USD 1576.00 million by 2032, with a CAGR of 7.03 % during the forecast period of 2025 to 2032.
With increasing globalization and digital disruption, the Equine X-Ray Solutions Market is expanding across multiple industries, . Market research data indicates that businesses in the Companion Animal…

Veterinary X-Ray Market Size, Analysis, Scope, Demand, Opportunities, Statistics
According to Data Bridge Market Research The global Veterinary X-Ray market size was valued at USD 915.19 million in 2024 and is projected to reach USD 1576.00 million by 2032, with a CAGR of 7.03 % during the forecast period of 2025 to 2032.
With increasing globalization and digital disruption, the Equine X-Ray Solutions Market is expanding across multiple industries, . Market research data indicates that businesses in the Companion Animal…
More Releases for CTMS
Key Trends Shaping the Future Clinical Trial Management System CTMS Market From …
What Is the Estimated Market Size and Growth Rate for the Clinical Trial Management System CTMS Market?
The clinical trial management system (CTMS) market has experienced rapid growth in recent years. It is expected to grow from $1.41 billion in 2024 to $1.61 billion in 2025, with a CAGR of 14.2%. Growth factors include increasing clinical trial complexity, a rise in global clinical trials, regulatory compliance demands, an increasing focus on…
Clinical Trial Management System (CTMS) Market: Growth, Trends, Opportunities, a …
Introduction
A Clinical Trial Management System (CTMS) is an integrated software platform designed to streamline and manage the planning, execution, and monitoring of clinical trials. It helps healthcare organizations, pharmaceutical companies, research institutions, and contract research organizations (CROs) efficiently oversee the complex and data-driven processes of clinical trials. The CTMS enables management of trial activities such as patient recruitment, data collection, regulatory compliance, budgeting, and reporting.
Clinical trials are a critical component…
Clinical Trial Management System (CTMS) Global Market Report 2024 - Clinical Tri …
"The Business Research Company recently released a comprehensive report on the Global Clinical Trial Management System (CTMS) Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
According to The Business Research Company's, The…
Clinical Trial Management System (CTMS) Market Scope & Future Opportunities Till …
An exclusive Clinical Trial Management System (CTMS) Market research report has been fabricated through the in depth analysis of the market dynamics across five regions including North America, Europe, South America, Asia-Pacific, Middle East and Africa. The segmentation of the market by components, end users, and region was done based on the thorough market analysis and validation through extensive primary inputs from industry experts (key opinion leaders of companies, and…
Latest Clinical Trial (CTMS) Market 2022 | Detailed Report
ReportsnReports publishes the report titled Clinical Trial (CTMS) that presents a 360-degree overview of the market under one roof. The report is developed with the meticulous efforts of an enthusiastic and experienced team of experts, analyts, and researchers that makes the report a valuable asset for stakeholders to make robust decisions. This report also provides an in-depth overview of product type, specification, technology, and production analysis considering vital factors like…
Clinical Trial Management System (CTMS) Market - Global Industry Insights 2024
Clinical Trials Management System is software that helps in managing and monitoring data related to clinical trials for pharmaceutical and biotechnology companies. The core objective of clinical trial management system is to maintain a centralized database which allows users to access data at any point of time. The need for these systems stems from the growth in clinical trials over the last decade and it has become essential to manage…
