Press release
US Software as a Medical Device Market Set for Explosive Growth to US$ 715.00 Million by 2033, Led by North America's 44.8% Market Share | Key Players - Viz.ai, Medtronic Plc, AliveCor
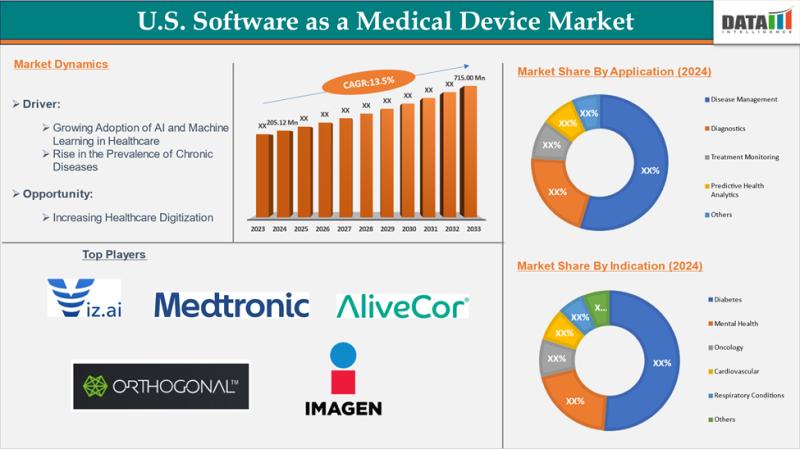
The US Software as a Medical Device (SaMD) market reached US$ 205.12 million in 2024 and is expected to reach US$ 715.00 million by 2033, growing at a CAGR of 13.5% during the forecast period 2025-2033.Market expansion is propelled by the rising prevalence of chronic diseases like diabetes, cardiovascular conditions, mental health disorders, and cancers, alongside healthcare provider shortages and demand for real-time, personalized care. Advancements in AI-powered clinical decision support, digital diagnostics, predictive analytics, and continuous monitoring solutions are accelerating adoption in digital healthcare transformation.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/us-software-as-a-medical-device-market?ram
Key Industry Developments
United States:
✅ February 2026: Siemens Healthineers launched an advanced AI-powered SaMD platform for real-time cardiac imaging analysis, enhancing diagnostic accuracy and workflow efficiency in U.S. hospitals through FDA-cleared machine learning algorithms.
✅ January 2026: Caranx Medical received FDA clearance for its pioneering AI software providing intra-operative guidance for Transcatheter Aortic Valve Implantation (TAVI), marking a breakthrough in real-time procedural support.
✅ November 2025: FDA issued expanded guidance on AI/ML-based SaMD lifecycle management, streamlining approvals for adaptive algorithms in diagnostics and predictive tools.
Asia Pacific / Japan:
✅ January 2026: Philips Japan introduced a cloud-based SaMD solution for remote neurology monitoring, incorporating wearable data integration for stroke prediction in aging populations.
✅ December 2025: GE Healthcare Japan advanced R&D in SaMD for oncology, launching an AI-driven radiotherapy planning software with real-time dose optimization.
Key Mergers and Acquisitions:
✅ Siemens & Dotmatics: Siemens AG announced the acquisition of U.S.-based R&D scientific software company Dotmatics for $5.1 billion, bolstering its Life Sciences portfolio and expanding presence in software for medical and industrial applications.
✅ Clearlake Capital & Modernizing Medicine: Clearlake Capital acquired a majority stake in healthcare software firm Modernizing Medicine (ModMed) for $5.3 billion, the largest healthcare LBO of 2025, enhancing electronic health records for over 160,000 U.S. physicians.
✅ Bain Capital & HealthEdge: Bain Capital acquired U.S. healthcare software firm HealthEdge from Blackstone for $2.6 billion, integrating generative AI to modernize systems for 115 health insurers covering 110 million lives.
✅ Waystar & Iodine Software: Waystar acquired Iodine Software for $1.25 billion, adding AI-driven documentation and reimbursement tools to support hospitals and healthcare providers in the U.S.
Key Players:
Viz.ai, Inc. | Medtronic Plc | AliveCor | Empatica | Bigfoot Biomedical | Digital Diagnostics Inc. | Imagen | Orthogonal
Strategic Leadership Report: Top 5 Players in US Software as a Medical Device Market 2026
-Viz.ai, Inc.: Launched Viz Assist, the first multimodal AI agent platform combining imaging and EHR data for autonomous patient identification, real-time insights, and clinical co-pilot functionality to accelerate critical care decisions.
-Medtronic Plc: Introduced the MiniMed 780G system with Instinct sensor integration, an FDA-cleared advanced diabetes management software providing automated insulin delivery and seamless remote monitoring via app-based technology.
-AliveCor: Received FDA clearance for Kardia AI V2, a sophisticated algorithm suite expanding personal ECG capabilities to detect a wider range of cardiac conditions beyond AFib for enhanced remote cardiology services.
-Empatica: Unveiled EmbraceMini, the world's smallest FDA-cleared actigraphy wearable with AI-driven remote monitoring software for clinical trials assessing epilepsy, sleep, stress, and other conditions unobtrusively.
-Bigfoot Biomedical: Secured FDA 510(k) clearance for the Android version of the Bigfoot Unity Mobile App, enabling intelligent connected insulin dosing support and diabetes management for a broader user base.
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=us-software-as-a-medical-device-market?ram
Market Drivers and Key Trends:
-AI-Powered Diagnostics: AI integration in SaMD enhances real-time disease detection and predictive analytics, improving diagnostic accuracy for chronic conditions like diabetes.
-Chronic Disease Rise: Increasing prevalence of chronic illnesses drives demand -for remote monitoring and personalized treatment via SaMD platforms.
-Regulatory Support: FDA's streamlined approvals for AI-driven SaMD accelerate innovation and market entry for digital health tools.
-Telemedicine Expansion: Proliferation of remote patient monitoring and home-care solutions boosts SaMD adoption amid healthcare accessibility needs.
-Market Hurdles: Stringent FDA regulations, data privacy concerns under HIPAA, and high validation costs for clinical efficacy constrain faster growth.
Regional Insights:
-North America: 44.8% (Largest share, driven by FDA regulatory support, advanced health IT infrastructure, and high investment in AI diagnostics).
-Asia Pacific: 21.6% CAGR projected (Fastest growing, fueled by smartphone penetration, digital health initiatives in China, India, and Japan).
-Europe: 21.3% CAGR projected (Strong growth from EU MDR implementation, DiGA reimbursements in Germany, and digital health reforms).
Market Opportunities & Challenges: US Software as a Medical Device Market 2026
-Opportunities: FDA's continuous-learning AI pathways accelerate SaMD approvals for real-time diagnostics and predictive analytics in chronic disease management. Telehealth integration and value-based care reimbursements enable seamless remote monitoring expansions, while streamlined 510(k) clearances support personalized treatment platforms for mental health and wearables.
-Challenges: Stringent cybersecurity mandates and data privacy regulations under HIPAA intensify compliance burdens, as interoperability gaps with legacy EHR systems slow adoption. High development costs for clinical validation and fragmented reimbursement policies across payers demand robust post-market surveillance strategies.
-Strategic Verdict: AI-driven diagnostic tools and telehealth-enabled remote monitoring platforms emerge as dominant 2026 growth vectors.
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/us-software-as-a-medical-device-market?ram
Market Segmentation Analysis:
-By Application
Disease Management holds 38% share, leading with AI-driven tools for chronic condition monitoring, adherence tracking, and real-time interventions to cut hospitalizations.
Diagnostics captures 25%, using imaging analysis and early detection algorithms for precise clinical insights.
Treatment Monitoring and Predictive Health Analytics split 25%, enabling remote alerts and personalized plans; Others take 12% for niche uses.
-By Indication
Diabetes dominates at 32%, powered by integrations with glucose monitors and glycemic prediction for Type 1/2 management.
Oncology follows at 14%, with tumor tracking and AI radiology for treatment adjustments.
Respiratory Conditions at 9% aids COPD/asthma monitoring; Cardiovascular, Mental Health, and Others share 45%.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription?ram
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTW
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release US Software as a Medical Device Market Set for Explosive Growth to US$ 715.00 Million by 2033, Led by North America's 44.8% Market Share | Key Players - Viz.ai, Medtronic Plc, AliveCor here
News-ID: 4397249 • Views: …
More Releases from DataM intelligence 4 Market Research LLP

Smart Healthcare Automation Market Set for Strong Growth to USD 69.06 Billion by …
The Smart healthcare automation market size stands at USD 44.75 billion in 2025 and is projected to reach USD 69.06 billion by 2030, advancing at a 9.07% CAGR over the forecast period. Rising adoption of AI-driven diagnostics, robotic process automation, and IoT-enabled medical devices is accelerating smart healthcare automation growth. Increasing demand for operational efficiency, cost reduction, real-time patient monitoring, and improved clinical outcomes further drives investment across hospitals, laboratories,…

Pharmaceutical Filtration Market Set for Strong Growth to USD 21.96 Billion by 2 …
The global Pharmaceutical filtration market size was estimated at USD 11.67 billion in 2022 and is projected to reach USD 21.96 billion by 2030, growing at a CAGR of 8.03% from 2023 to 2030. Growth is driven by rising biopharmaceutical production, increasing vaccine manufacturing, stringent regulatory requirements for sterile processing, expanding generic drug demand, and growing R&D investments. Additionally, advancements in filtration technologies and heightened focus on contamination control in…

E-Commerce Packaging Market Set for Explosive Growth to USD 392.85 Billion by 20 …
The global E-Commerce Packaging Market size was valued at USD 106.46 billion in 2025 and is projected to reach USD 392.85 billion by 2033, growing at a CAGR of 17.9% from 2026 to 2033. Rapid expansion of online retail, increasing smartphone and internet penetration, and changing consumer buying behavior are driving demand for secure, sustainable, and customized packaging. Growth in cross-border trade, same-day delivery models, and eco-friendly packaging innovations further…
Sickle Cell Disease and β-Thalassemia Gene Therapy Market Set for Explosive Gro …
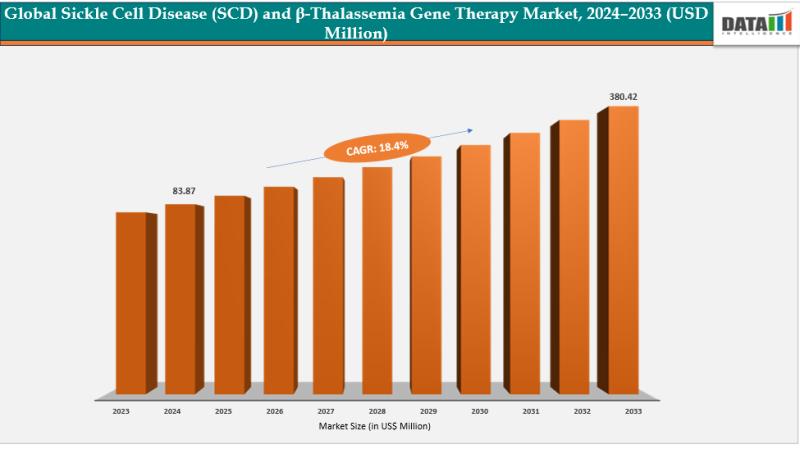
The Global Sickle Cell Disease and β-thalassemia gene therapy market size was US$ 83.87 Million in 2024 and is expected to reach US$ 380.42 Million by 2033, growing at a CAGR of 18.4% during the forecast period 2025-2033.
Market expansion is propelled by advancements in gene-editing technologies like CRISPR/Cas9 and lentiviral vectors, enabling precise genetic corrections and potential cures. Rising clinical trials demonstrate long-term safety and efficacy, while regulatory approvals for…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…