Press release
Generic Drug Manufacturing Plant DPR & Unit Setup - 2026: Machinery Cost, CapEx/OpEx, ROI, Raw Materials
The global generic drug manufacturing industry is witnessing robust growth driven by the growing demand for affordable medicines, increasing prevalence of chronic diseases, and rising penetration of healthcare services across emerging economies. At the heart of this expansion lies a critical pharmaceutical segment-generic drugs. As healthcare systems worldwide focus on cost containment, broader coverage, and ensuring equitable access to essential medicines, establishing a generic drug manufacturing plant presents a strategically compelling business opportunity for investors seeking to capitalize on this growing and essential market.Market Overview and Growth Potential
The global generic drug market demonstrates a strong growth trajectory, valued at USD 411.02 Billion in 2025. According to IMARC Group's comprehensive market analysis, the market is expected to reach USD 713.10 Billion by 2034, exhibiting a CAGR of 5.7% from 2026 to 2034. This sustained expansion is driven by the growing demand for affordable medicines, increasing prevalence of chronic diseases, and rising penetration of healthcare services across emerging economies.
Generic drugs are pharmaceutical products which doctors can use to treat patients because they deliver the same therapeutic effects as brand-name medications through identical dosage forms and drug strengths and administration methods and product quality and safety and treatment effectiveness. The drugs include all active pharmaceutical ingredients which reference drugs contain and manufacturers produce the products according to strict regulatory standards which guarantee that the drugs will have the same therapeutic effect as reference drugs. Pharmaceutical companies create generic drugs after the patent protection period ends because this process allows them to produce affordable treatment methods which maintain effective medical results.
The global generic drug industry market is primarily driven by the rising healthcare expenditure, expanding access to medicines, and increasing acceptance of generics among healthcare professionals and patients. The demand for low-cost therapeutic alternatives has intensified as healthcare systems worldwide focus on cost containment and broader coverage. Moreover, emerging economies are witnessing rapid expansion in pharmaceutical consumption due to urbanization, higher disease awareness, and improvements in healthcare infrastructure. As per the Press Information Bureau (PIB) sales insights from Pharmarack and export figures from the Directorate General of Commercial Intelligence and Statistics, the total value of generic medicines produced in India increased to 26%, rising from Rs. 3,22,116 crore in FY2022-2023 to Rs. 4,06,047 crore in FY2024-2025. Ongoing patent expirations and increasing focus on essential medicines are expected to sustain long-term industry growth in the coming years.
Plant Capacity and Production Scale
The proposed generic drug manufacturing facility is designed with an annual production capacity ranging between 1 - 5 billion tablets/capsules, enabling economies of scale while maintaining operational flexibility. This capacity range allows manufacturers to cater to diverse market segments-from pharmaceuticals, hospitals and clinics, and retail pharmacies to government and public healthcare systems-ensuring steady demand and consistent revenue streams across multiple industry verticals. The facility is designed to produce a broad portfolio of therapeutic categories including chronic disease management, infectious diseases, pain management, cardiovascular disorders, and metabolic conditions.
Request for a Sample Report: https://www.imarcgroup.com/generic-drug-manufacturing-plant-project-report/requestsample
Financial Viability and Profitability Analysis
The generic drug manufacturing business demonstrates healthy profitability potential under normal operating conditions. The financial projections reveal:
Gross Profit Margins: 55-65%
Net Profit Margins: 25-35%
These margins are among the most attractive across the manufacturing sector, supported by stable demand across the pharmaceutical and healthcare sectors, significant value-added positioning relative to branded drugs, and the critical nature of generic drugs in national healthcare systems worldwide. The project demonstrates strong return on investment (ROI) potential, making it an attractive proposition for both new pharmaceutical entrants and established chemical manufacturers looking to diversify into the high-value specialty generic medicines sector.
The profitability breakdown across the five-year financial projection period is presented below:
Operating Cost Structure
Understanding the operating expenditure (OpEx) is crucial for effective financial planning and cost management. The cost structure for a generic drug manufacturing plant is primarily driven by:
Raw Materials: 40-50% of total OpEx
Utilities: 10-15% of OpEx
Other Expenses: Including labor, packaging, transportation, maintenance, depreciation, and taxes
Raw materials constitute the largest single portion of operating costs, with Active Pharmaceutical Ingredients (APIs) being the primary input material. Additional key raw materials include excipients, packaging materials such as blisters and bottles, and water for injection. Establishing long-term contracts with reliable API and excipient suppliers helps mitigate price volatility and ensures consistent raw material supply, which is critical given that API price fluctuations represent the most significant cost factor in generic drug manufacturing.
Capital Investment Requirements
Setting up a generic drug manufacturing plant requires substantial capital investment across several critical categories:
Land and Site Development:
Selection of an optimal location with strategic proximity to key raw material suppliers of APIs, excipients, packaging materials such as blisters and bottles, and water for injection. Proximity to target pharmaceutical markets will help minimize distribution costs. The site must have robust infrastructure, including reliable transportation, utilities, and waste management systems. Compliance with local zoning laws, pharmaceutical manufacturing regulations, and environmental regulations must also be ensured.
Machinery and Equipment:
The largest portion of capital expenditure (CapEx) covers specialized manufacturing equipment essential for pharmaceutical-grade production. Key machinery includes:
• Mixers and granulators: for precise blending and granulation of API and excipient formulations to achieve uniform composition
• Tablet presses: for compressing granulated material into tablets of accurate weight, hardness, and dissolution profile
• Capsule filling machines: for filling hard or soft gelatin capsules with precise doses of active and inactive ingredients
• Coating systems: for applying functional or aesthetic coatings to tablets for taste masking, moisture protection, and controlled release
• Blister packaging lines: for forming, filling, and sealing primary blister packs to protect finished dosage forms
• Laboratory instruments: for in-process and finished product quality testing including dissolution, HPLC, and stability testing
• Water for injection (WFI) systems: for producing pharmaceutical-grade purified water required across manufacturing operations
• HVAC and cleanroom systems: for maintaining GMP-compliant controlled environments throughout the production facility
Civil Works:
Building construction, factory layout optimization, and infrastructure development designed to enhance workflow efficiency, ensure workplace safety, and minimize material handling complexities throughout the production process. The layout should be optimized with separate areas for raw material storage, dispensing room, granulation and mixing zone, compression or encapsulation section, coating unit, quality control laboratory, packaging line, finished goods warehouse, utility block, effluent treatment area, and administrative block.
Other Capital Costs:
Pre-operative expenses, machinery installation costs, GMP certification and regulatory compliance costs, initial working capital requirements, validation and qualification expenses for equipment and processes, and contingency provisions for unforeseen circumstances during plant establishment.
Speak to Analyst for Customized Report: https://www.imarcgroup.com/request?type=report&id=7875&flag=C
Major Applications and Market Segments
Generic drug products find extensive applications across diverse market segments, demonstrating their critical importance and broad therapeutic reach:
Hospitals and Clinics:
Widely prescribed for inpatient and outpatient treatments due to their cost-effectiveness and regulatory approval. Generic drugs form the backbone of hospital formularies, enabling healthcare institutions to deliver essential care across cardiovascular, infectious disease, metabolic, and pain management categories at significantly lower cost than branded equivalents.
Retail Pharmacies:
Pharmacies dispense generic medicines as substitutes for branded drugs, improving affordability and medication adherence among patients. Retail pharmacy dispensing of generics is supported by substitution policies, patient education campaigns, and physician prescribing incentives across major markets.
Government Healthcare Programs:
Public health initiatives rely heavily on generic drugs to support national immunization, disease control, and subsidized medicine distribution schemes. Government procurement of generics represents one of the largest single demand sources globally, driven by policy commitments to essential medicines access and healthcare cost containment.
Chronic Disease Management:
Extensively used for long-term therapies such as diabetes, hypertension, and cardiovascular care. The rising global burden of non-communicable diseases is creating sustained and growing demand for affordable generic treatment options across all age groups and geographies.
Why Invest in Generic Drug Manufacturing?
Several compelling factors make generic drug manufacturing an attractive investment opportunity:
Essential Healthcare Backbone:
Generic drugs form the foundation of modern healthcare systems because they provide essential medicines at affordable prices which remain available throughout the globe in both developed and emerging markets. This structural criticality creates a durable, recession-resistant demand profile unmatched in most other manufacturing sectors.
High and Sustained Demand:
The increasing burden of chronic and lifestyle diseases together with the rising number of elderly people creates a constant requirement for generic medicines which treat all medical conditions. This demographic and epidemiological megatrend provides long-term visibility into market demand for manufacturers.
Patent Expiry-Driven Opportunities:
The ongoing process of patenting all major pharmaceutical products creates multiple chances for international generic companies to enter new markets. Each major drug patent expiration represents a significant revenue opportunity for compliant generic manufacturers ready to supply equivalent therapies at substantially lower price points.
Favorable Policy Environment:
The government programs which support affordable healthcare together with their price control policies and domestic drug manufacturing initiatives create a strong foundation for the growth of generic medication production. Regulatory frameworks in major markets continue to streamline generic approvals through abbreviated new drug application pathways.
Global Supply Chain Integration:
Manufacturers can supply their products to domestic markets and regulated export markets because they prefer suppliers who combine reliability with compliance and low costs. India's position as the global pharmacy, supplying over 60 countries with generic medicines, exemplifies the export opportunity available to compliant manufacturers.
Manufacturing Process Excellence
The generic drug manufacturing process involves several precision-controlled stages under strict Good Manufacturing Practice (GMP) compliance:
• Formulation Development: APIs and excipients are selected, tested, and formulated to achieve the target therapeutic profile and bioequivalence
• Granulation or Blending: Raw materials are granulated or blended using mixers and granulators to achieve uniform particle size distribution and content uniformity
• Compression or Encapsulation: Granulated blends are compressed into tablets using high-speed tablet presses or filled into capsules using capsule filling machines
• Coating: Tablets undergo film coating or enteric coating in coating systems to achieve desired dissolution, stability, and patient compliance properties
• In-Process Quality Testing: Samples are drawn at each stage for weight variation, hardness, friability, dissolution, and content uniformity testing
• Inspection: Finished dosage forms undergo visual inspection for defects, foreign particles, and compliance with dimensional specifications
• Packaging: Products are blister-packed or bottle-packed on automated packaging lines and labeled with regulatory-compliant product information
Buy Now: https://www.imarcgroup.com/checkout?id=7875&method=2175
Industry Leadership
The global generic drug industry is led by established pharmaceutical manufacturers with extensive production capabilities and diverse therapeutic portfolios. Key industry players include:
• Cipla
• Lupin
• Teva Pharmaceuticals
• Sandoz (Novartis)
• Mylan / Viatris
• Sun Pharmaceutical Industries
• Dr. Reddy's Laboratories
These companies serve diverse end-use sectors including pharmaceuticals, hospitals and clinics, retail pharmacies, and public healthcare systems, demonstrating the broad market applicability of generic drug products across therapeutic categories globally.
Recent Industry Developments
October 2025: Australia-based AI-driven drug developer Algorae Pharmaceuticals signed a binding term sheet with India's Cadila Pharmaceuticals for a proposed license and supply partnership. The collaboration will support the launch of two generic medicines across Australia and New Zealand, targeting cardiovascular and metabolic therapies. The partners will now move toward a definitive agreement and regulatory filings with Australia's TGA.
June 2025: Hikma Pharmaceuticals USA announced plans to invest USD 1 billion by 2030 to expand its US manufacturing and research footprint. Building on more than three decades of operations in the country, the investment will strengthen domestic production of essential generic medicines, enhance research and development capabilities, and support supply reliability for the US healthcare system amid growing demand.
April 2025: Alembic Pharmaceuticals Limited received final USFDA approval for its Abbreviated New Drug Application for Carbamazepine Tablets USP, 200 mg. Therapeutically equivalent to Novartis' Tegretol, the drug treats seizure disorders and trigeminal neuralgia and addresses a USD 32 million US market, strengthening Alembic's expanding ANDA portfolio.
Conclusion
The generic drug manufacturing sector presents a strategically positioned investment opportunity at the intersection of affordable healthcare, essential medicines access, and sustained pharmaceutical demand. With exceptional profit margins ranging from 55-65% gross profit and 25-35% net profit, strong market drivers including growing demand for affordable medicines, the rising prevalence of chronic diseases, ongoing patent expirations unlocking new market opportunities, and supportive government policies promoting domestic drug manufacturing and essential medicines access, establishing a generic drug manufacturing plant with a production capacity of 1-5 billion tablets/capsules per year offers significant potential for long-term business success and sustainable returns. The combination of the essential healthcare backbone role of generic drugs, the patent expiry-driven pipeline of new market opportunities, favorable regulatory environments in major export markets, and India's growing global position as the pharmacy of the world creates a compelling value proposition for pharmaceutical investors committed to quality manufacturing, GMP compliance, and operational excellence.
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers create a lasting impact. The company excels in understanding its clients' business priorities and delivering tailored solutions that drive meaningful outcomes. IMARC Group provides a comprehensive suite of market entry and expansion services, including market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No: (D) +91 120 433 0800
United States: (+1-201-971-6302)
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Generic Drug Manufacturing Plant DPR & Unit Setup - 2026: Machinery Cost, CapEx/OpEx, ROI, Raw Materials here
News-ID: 4393971 • Views: …
More Releases from IMARC Group
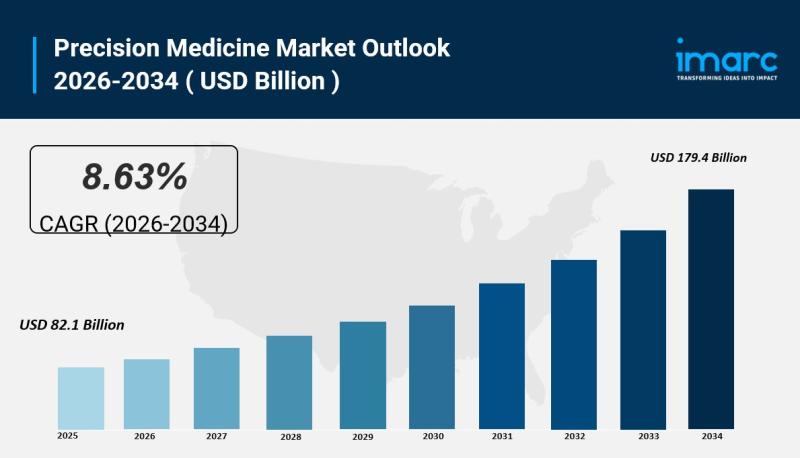
Precision Medicine Market Size to Surpass USD 179.4 Billion by 2034 | At CAGR 8. …
IMARC Group has recently released a new research study titled "Precision Medicine Market Size, Share, Trends and Forecast by Product, Technology, Application, End User, and Region, 2026-2034", offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios.
Market Overview
The global precision medicine market size reached USD 82.1 Billion in 2025 and is expected to grow to USD 179.4…

Edible Oil Manufacturing Plant DPR 2026: Cost Structure, Production Process & RO …
The global food and beverage industry is experiencing transformative growth driven by rising health-conscious consumer preferences, the expanding food processing industry, and increasing demand in emerging economies. At the forefront of this essential food ingredients revolution stands edible oil-a versatile fat derivative valued for its critical role in cooking, frying, food preparation, and as an ingredient in packaged foods across food and beverage, restaurant and catering, health and wellness, and…
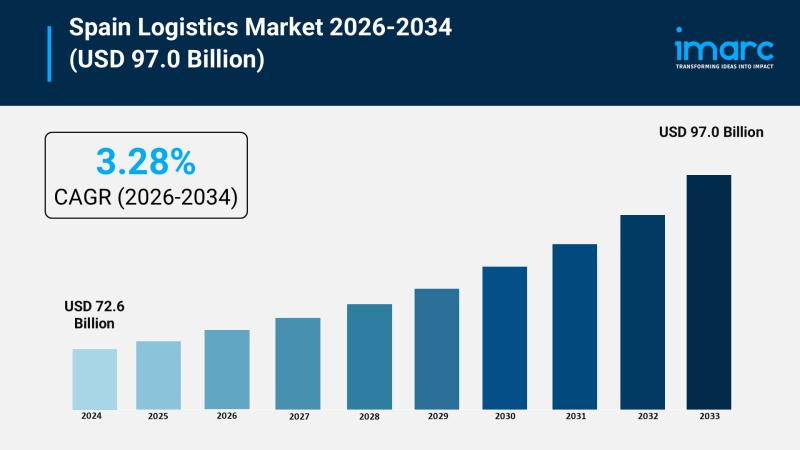
Spain Logistics Market Growth Forecast USD 72.6 Billion in 2025 to USD 97 Billio …
Market Overview
The Spain logistics market size reached USD 72.6 Billion in 2025 and is forecasted to grow to USD 97.0 Billion by 2034. The market is expected to expand at a CAGR of 3.28% during the forecast period 2026-2034. Driving factors include increasing e-commerce users, growing demand for warehousing, urban logistics, ongoing technological advancements, and rising focus on sustainability initiatives.
Study Assumption Years
• Base Year: 2025
• Historical Year/Period: 2020-2025
• Forecast Year/Period: 2026-2034
Spain Logistics Market…

Oral Rehydration Salt (ORS) Manufacturing Cost Analysis DPR 2026: CapEx/OpEx Ana …
The global oral rehydration salt (ORS) manufacturing industry is witnessing robust growth driven by the rapidly expanding healthcare sector and increasing demand for effective dehydration management solutions. At the heart of this expansion lies a critical essential medicine-oral rehydration salt. As healthcare systems transition toward preventive care and community-based treatment approaches, establishing an ORS manufacturing plant presents a strategically compelling business opportunity for entrepreneurs and pharmaceutical investors seeking to capitalize…
More Releases for Generic
Prominent Generic Oncology Drugs Market Trend for 2025: Novel Formulations Trans …
Which drivers are expected to have the greatest impact on the over the generic oncology drugs market's growth?
The upward trend in cancer incidence is predicted to fuel the expansion of the generic oncology drugs market. Cancer, characterized by unregulated cell proliferation affecting numerous organs, results in considerable morbidity and mortality globally. The provision of affordable generic oncology drugs is an instrumental factor in cancer care, expanding access to vital treatments,…
Generic Theater Presents Flyin' West
NORFOLK, VA (August 2023) - Generic Theater inaugurates its 43rd season with Flyin' West, written by award-winning playwright and New York Times bestselling author Pearl Cleage. Terrance Afer-Anderson directs this production, running weekends September 8th - October 1st at Generic Theater, Norfolk's underground theater located in the basement of Chrysler Hall.
Following the end of the Civil War, many former slaves took advantage of The Homestead Act and went West to…
Generic Injectables Market growth is attributed to the increasing demand for Onc …
According to Precision Business Insights (PBI), the latest report, the generic injectables market will be worth USD 22.0 billion in 2022, growing at an 11.0% CAGR from 2022 to 2028. The global generic injectables market is segmented into the following types: Product Type (Monoclonal Antibodies, Cytokines, Insulin, Peptide Hormones, Blood Factors, Immunoglobulins, Peptide Antibiotics, Vaccines, and Others), Indication (Diabetes, Cancer, Cardiovascular Diseases, Musculoskeletal, CNS, Infections, and Others), Distribution Channel (Hospital…
A Demand On Generic Drugs Market And The Need To Push The Market Of Generic Drug …
Global Generic Drugs Market
A generic drug is pharmaceutical drug, which is bio-equivalent to a branded drug in all forms such as route of administration, strength, dosage, quality, intended use and performance. Generic drugs are usually approved after patent expiration of patent drugs. Generic drugs are safe, effective and FDA approved. The global market is filled with 44% of generic drugs.
The global generic drugs market is driven by the rise of…
Generic Drug Market: Global Generic Drug Share to Reach USD 380.60 Billion by 20 …
Zion Market Research has published a new report titled “Generic Drug Market by Brand (Pure Generic and Branded Generic) for Central Nervous System (CNS), Cardiovascular, Dermatology, Oncology, Respiratory and Others Therapeutic Applications - Global Industry Perspective, Comprehensive Analysis and Forecast, 2015 – 2021”. According to the report, the global generic drug market accounted for around USD 200.20 billion in 2015 and is expected to reach approximately USD 380.60 billion by…
Generic Drugs: Global Collaboration Opportunities
Collaboration is the key to growth
With the recent economic turmoil affecting many markets around the world, the generic and branded sectors face similar problems.
At one time the generic sector was seen as a valuable way to bring effective products to a wider audience. This, allied to the rising cost of new advanced branded products, led to rapid expansion of the generics sector. Even markets traditionally based in the provision of…
