Press release
Rapid Medical Diagnostics Market to Reach US$ 45.28 Billion by 2033 at 12.8% CAGR; North America Leads with 36% Share - Key Players: Abbott Laboratories, Roche Diagnostics, Siemens Healthineers
The Rapid Medical Diagnostics Market was valued at USD 15.12 billion in 2024 and is projected to reach USD 45.28 billion by 2033, expanding at a CAGR of 12.8% during the forecast period from 2025 to 2033. This robust growth is driven by the rising global burden of infectious diseases, demand for prompt clinical decision making, increased need for point-of-care testing, and growing adoption of rapid diagnostics for emergency care and community health screening. Rapid medical diagnostics facilitate quicker detection of pathogens, biomarkers, and critical health conditions, enabling early intervention and improved patient outcomes across hospitals, clinics, and decentralized healthcare settings.Technological advancements in immunoassays, molecular diagnostics, microfluidics, and biosensor platforms are enhancing test sensitivity, specificity, and portability. The integration of artificial intelligence, mobile connectivity, and automated assay interpretation is further expanding capabilities for decentralized testing. Recent industry momentum includes increased deployment of rapid diagnostics for respiratory infections, sexually transmitted diseases, cardiovascular biomarkers, and metabolic disorders, alongside expanded use of near-patient testing in rural and resource-limited regions. These innovations are positioning rapid medical diagnostics as a cornerstone of next-generation healthcare delivery, improving accessibility, reducing turnaround times, and supporting proactive disease management worldwide.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/rapid-medical-diagnostics-market?sai-v
Rapid Medical Diagnostics Market refers to the industry focused on the development and commercialization of fast, point-of-care diagnostic technologies that enable quick detection of diseases and health conditions for timely clinical decision-making.
Key Developments
✅ February 2026: In the United States, Abbott Laboratories expanded commercial availability of its latest rapid point-of-care diagnostic tests, including updated panels for respiratory pathogens that deliver results in under 15 minutes to support urgent care and emergency settings.
✅ January 2026: Across Europe (United Kingdom and Germany), Roche Diagnostics introduced an enhanced version of its rapid immunoassay platform for infectious disease screening, offering improved sensitivity and expanded test menus for hospitals and clinics.
✅ December 2025: In Asia Pacific (India and Southeast Asia), Trivitron Healthcare reported increased deployment of rapid diagnostic solutions for infectious diseases, including advanced immunochromatographic test kits in primary care and community health settings.
✅ November 2025: In North America and Europe, Siemens Healthineers launched upgraded rapid diagnostic assays integrated with digital analytics to support real-time clinical decision making and faster triage in emergency departments.
✅ October 2025: In global diagnostics markets, QuidelOrtho Corporation expanded production capacity for its rapid antigen and antibody tests to meet continued demand in outpatient clinics and retail health services.
✅ September 2025: In Africa and Latin America, public health programs increased use of rapid diagnostic tests for malaria, HIV, and other endemic diseases as part of strengthened community health screening and early treatment initiatives.
Key Players
Abbott Laboratories | Bio-Rad Laboratories | AccessBio Inc. | AccuBioTech Co. Ltd. | Quidel Corporation | Meridian Bioscience Inc. | OraSure Technologies Inc. | Thermo Fisher Scientific Inc. | Becton Dickinson and Company | Trinity Biotech Plc. | Others
Buy Now & Unlock 360° Market Intelligence:https://www.datamintelligence.com/buy-now-page?report=rapid-medical-diagnostics-market?sai-v
(Single User Report: USD 4350 & One Year Database Subscription: USD 12K
Market Drivers
Rising Demand for Point of Care Testing: Need for quick diagnosis in emergency rooms, clinics, and home settings is accelerating adoption of rapid diagnostic solutions.
Growing Burden of Infectious and Chronic Diseases: Increasing prevalence of conditions such as respiratory infections, diabetes, and cardiovascular diseases is driving frequent testing.
Need for Early Disease Detection and Treatment: Rapid diagnostics enable timely clinical decisions, improving patient outcomes and reducing healthcare costs.
Advancements in Portable and User Friendly Devices: Miniaturized analyzers, lateral flow assays, and handheld molecular tools are expanding accessibility.
Expansion of Telehealth and Home Healthcare: Remote care models are increasing reliance on self testing and decentralized diagnostic technologies.
Industry Developments
Launch of Next Generation Rapid Test Kits: Companies are introducing highly sensitive antigen, antibody, and molecular assays with faster turnaround times.
Integration with Digital Health Platforms: Connectivity with mobile apps and cloud systems enables real time reporting, monitoring, and data analytics.
Growth of Multiplex and Molecular Rapid Testing: Advanced platforms now detect multiple pathogens or biomarkers from a single sample.
Strategic Collaborations and Regulatory Approvals: Partnerships between diagnostics firms and healthcare providers are accelerating commercialization and adoption.
Focus on Affordable and Scalable Testing Solutions: Manufacturers are prioritizing low cost, mass producible diagnostics for global public health use.
Regional Insights
North America - Holds 36% share: Advanced healthcare infrastructure, strong diagnostic innovation, and high testing awareness drive market leadership.
Europe - Holds 27% share: Supportive regulatory systems, widespread screening programs, and aging population sustain steady growth.
Asia Pacific - Holds 26% share: Large patient population, expanding healthcare access, and rising investment in diagnostics accelerate demand.
Latin America - Holds 6% share: Improving laboratory networks and infectious disease monitoring support gradual expansion.
Middle East and Africa - Holds 5% share: Growing healthcare investment and need for accessible diagnostics contribute to emerging adoption.
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/rapid-medical-diagnostics-market?sai-v
Key Segments
By Technology
Lateral flow immunoassays account for a dominant share due to their rapid results, ease of use, portability, and suitability for point of care and home based testing across multiple conditions. Solid phase assays maintain strong clinical relevance supported by higher sensitivity, laboratory compatibility, and broader diagnostic applications. Agglutination assays continue to be used for quick qualitative detection in clinical and laboratory environments because of their simplicity and cost effectiveness. Microfluidic chips are emerging rapidly as advanced diagnostic platforms offering miniaturization, reduced sample volume, faster processing, and potential integration with digital health technologies.
By Type
Infectious diseases represent the largest segment driven by ongoing need for rapid screening, outbreak management, and early diagnosis across healthcare settings. Toxicology testing holds significant demand supported by drug monitoring, substance abuse detection, and workplace screening requirements. Cardiovascular and gastrointestinal disorder diagnostics are expanding steadily due to rising chronic disease prevalence and increasing adoption of early detection and monitoring solutions.
By End Users
Hospitals dominate usage because of high patient volumes, availability of diagnostic infrastructure, and demand for rapid clinical decision making. Clinics maintain a substantial share supported by outpatient testing, routine screening, and decentralized healthcare delivery. Self care is the fastest growing segment driven by rising consumer awareness, availability of home testing kits, and growing preference for convenient, private, and real time health monitoring.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Rapid Medical Diagnostics Market to Reach US$ 45.28 Billion by 2033 at 12.8% CAGR; North America Leads with 36% Share - Key Players: Abbott Laboratories, Roche Diagnostics, Siemens Healthineers here
News-ID: 4393088 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
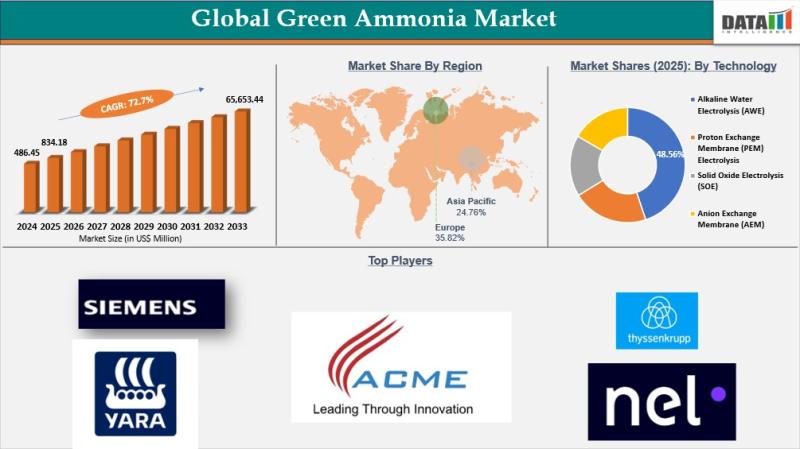
Green Ammonia Market to Reach US$ 65,653.44 Million by 2033 at 72.7% CAGR; Asia …
The Green Ammonia Market reached USD 486.45 million in 2024, rising to USD 834.18 million in 2025, and is projected to surge to USD 65,653.44 million by 2033, expanding at a CAGR of 72.7% during the forecast period from 2026 to 2033. This extraordinary growth reflects the accelerating global transition toward low carbon energy systems and sustainable agriculture inputs. Green ammonia, produced using renewable powered electrolysis and nitrogen synthesis, is…

Adhesives and Sealants Market to hit $ 119.9 billion by 2031 | Major Players 202 …
The Global Adhesives and Sealants Market reached US$ 78.1 billion in 2023 and is projected to reach US$ 119.9 billion by 2031, growing at a CAGR of 5.5% during the forecast period 2024-2031.
DataM Intelligence unveils exclusive insights into the Global Adhesives and Sealants Market 2026, highlighting technological innovations in high-performance formulations, increasing demand from construction and automotive sectors, and rising adoption of eco-friendly and specialty adhesives and sealants across industrial…

Control Moment Gyroscope (CMG) Market Growth Analysis (2026-2033) | Asia-Pacific …
Market Size and Growth
control moment gyroscope (CMG) market to reached US$ 1.58 billion in 2024, rising to US$ 1.70 billion in 2025, and is expected to reach US$ 3.05 billion by 2033, growing at a strong CAGR of 7.58% during the forecast period from 2026 to 2033
✅ Asia-Pacific is expected to be the fastest-growing region in the Control Moment Gyroscope (CMG) Market, accounting for approximately 29.5% of the market by…

Future of North & Central America Medical Ambulances Market (2026 ): Holds High …
Market Size and Growth
North & Central America Medical Ambulances Market size to hit at a CAGR during the forecast period 2024-2031
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/north-and-central-america-medical-ambulances-market?kb
North & Central America Medical Ambulances Market is a vital segment of the emergency medical services (EMS) ecosystem, encompassing ground ambulances, air ambulances, and associated equipment/services for transporting patients in medical emergencies and non-emergency scenarios.…
More Releases for Rapid
UYEE Rapid Tooling Announces Expert CNC Machining and Rapid Prototyping Services
UYEE Rapid Tooling Co., Ltd, a trusted provider since 2005, announces its advanced capabilities in high-speed CNC machining, rapid prototyping, and low volume manufacturing solutions.
UYEE Rapid Tooling Co., Ltd, a dedicated leader in manufacturing services since 2005, today highlighted its comprehensive suite of services designed to meet the demanding needs of modern product development and production. With a strong commitment to customer success, UYEE specializes in delivering high-speed CNC machining,…
Rapid Infuser Market - Swift Restoration, Enhanced Recovery: Rapid Infuser Optim …
Newark, New Castle, USA: The "Rapid Infuser Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Rapid Infuser Market: https://www.growthplusreports.com/report/rapid-infuser-market/8896
This latest report researches the industry structure, sales, revenue,…
Rapid Infuser Market - From Emergency to Efficiency: Advancing Patient Outcomes …
Newark, New Castle, USA - new report, titled Rapid Infuser Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Rapid Infuser market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Rapid Infuser market. The report offers an overview of the market, which…
Rapid Pregnancy Tests Market: Size & Trends Shows a Rapid Growth by 2027
The pregnancy test kit confirms pregnancy by detecting the level of human chorionic gonadotropin (HCG) in the urine. The growing demand for an easy and convenient way to get faster results, and the easy availability of pregnancy kits through various distribution channels, including pharmacies/pharmacies, online stores, etc., is increasing the demand for pregnancy test kits.
(Get 15% Discount on Buying this Report)
A full report of Global Rapid Pregnancy Tests Market is…
Rapid Machining Launches New Service
Rapid Machining, one of the largest prototype machining manufacturers in the United States, is excited to announce a new standard 5 day lead time service for lathe parts. Responding to customer demand, Rapid Machining purchased new equipment and reconfigured the manufacturing floor to make this lead-time reduction possible from a standard 7 day lead time. The new service is currently available for lathe parts with a variety of materials and…
RAPID: Lockheed Martin Approved
Nashua, NH – April 4, 2016 – RAPID was announced as an approved vendor for Lockheed Martin as of April 2016. RAPID has been working with Lockheed Martin divisions for a number of years, but the addition to the Approved Vendor list will strengthen and build upon the existing relationship.
Lockheed Martin is an aerospace, defense, security, and advanced technology company that employs over 116,000 professionals worldwide. Lockheed Martin has…