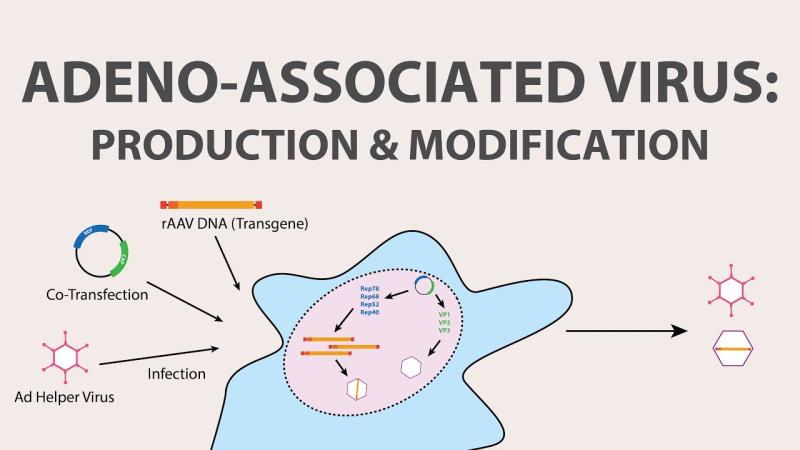
Press release
Adeno Associated Virus Vectors Manufacturing Market Set for Explosive Growth to US$ 9.98 Billion by 2034, Led by North America's 49.82% Market Share
The Adeno Associated Virus Vector Manufacturing Market is valued at around US$ 1.52 billion in 2025 and is projected to reach approximately US$ 9.98 billion by 2034, registering a strong CAGR of about 20.3% during 2025-2034.Market growth is driven by the rapid expansion of gene therapy pipelines, rising approvals of AAV‐based therapies, and increasing demand for GMP‐grade viral vectors from both clinical and commercial‐scale programs. Advancements in scalable suspension and HEK293/Sf9 production platforms, growing investment in rare disease and neurological disorder research, and the use of AAV vectors in vaccines and in vivo gene delivery are further accelerating market expansion.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/adeno-associated-virus-vectors-manufacturing-market?ram
Key Industry Developments
United States:
✅ February 2026: Sarepta Therapeutics expanded its Gene Therapy Center of Excellence with a new AAV vector production suite, incorporating automated perfusion bioreactors for higher yields and purity in DMD gene therapy manufacturing.
✅ January 2026: Forge Biologics launched a next-generation AAV8 vector platform with enhanced capsid engineering for improved liver tropism and reduced immune response.
✅ November 2025: Thermo Fisher Scientific introduced the Gibco AAV Max Helper System, a one-step transfection kit optimized for high-titer AAV production in the U.S. market.
Asia Pacific / Japan:
✅ January 2026: WuXi AppTec opened a new 50,000 sq ft AAV manufacturing facility in Tokyo, featuring single-use bioreactors for serotypes 5 and 9 used in ophthalmology trials.
✅ December 2025: Lonza Japan announced R&D advancements in plasmid-free AAV production using insect cell platforms, reducing costs by 40% for APAC clients.
✅ October 2025: Takara Bio launched a novel AAV capsid library screening service in Shiga, enabling customized vectors with superior neuronal transduction for Parkinson's research.
Strategic Mergers and Acquisitions:
✅ Thermo Fisher acquired Vigene Biosciences for $785 million in February 2025, enhancing AAV production with a high-yield suspension cell culture platform and expanding CDMO capabilities in the adeno-associated virus vectors market.
✅ Oxford Biomedica formed a strategic partnership with Eli Lilly in April 2025 for commercial AAV manufacturing, supporting neurological disease programs with potential $450 million revenue over five years.
Key Players:
Thermo Fisher Scientific Inc. | Oxford Biomedica PLC | Lonza Ltd | uniQure N.V. | FUJIFILM Diosynth Biotechnologies | SIRION Biotech GmbH | Curigin Inc. | Pfizer Inc. | GenScript Biotech Corp | Vector Biolabs
Strategic Leadership Report: Top 5 Players in AAV Vectors Manufacturing Market 2026
-Thermo Fisher Scientific Inc.: Launched the CTS AAV-MAX System, an all-in-one production platform with Gibco media, gene therapy kits, and POROS CaptureSelect AAV affinity resins including AAV8, AAV9, and AAVX variants for scalable purification and high-efficiency downstream processing.
-Oxford Biomedica PLC: Enhanced the inAAVateTM platform with optimized upstream processes via new transfection materials and parameter tuning, achieving over 2e15 vg/L titers across multiple serotypes and 10X bioreactor yields above industry averages using dual transfection in proprietary cell lines.
-Lonza Ltd: Developed a proprietary suspension HEK293 stable producer cell line platform, enabling helper virus-free AAV production with Xcite® transient and stable systems to optimize scalability, speed clinical advancement, and boost productivity for commercial gene therapies.
-uniQure N.V.: Advanced its insect cell-based large-scale AAV manufacturing platform, demonstrating capabilities to support expanded gene therapy pipelines with high yields suitable for broader patient populations and commercial supply needs.
-FUJIFILM Diosynth Biotechnologies: Expanded AAV vector services with integrated process development for high-titer production, featuring suspension cell culture optimizations and chromatography solutions to streamline scalability from clinical to commercial phases.
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=adeno-associated-virus-vectors-manufacturing-market?ram
Market Drivers and Key Trends:
-Gene Therapy Surge: Rising approvals and clinical pipelines for AAV-based treatments targeting genetic disorders and rare diseases are accelerating manufacturing demand.
-Scalable Production Advances: Shift to suspension cell cultures, HEK293 platforms, and automation boosts yields while cutting costs for commercial-scale output.
-Biopharma Investments: Partnerships with CDMOs and heavy funding from firms like Thermo Fisher enhance infrastructure for high-purity AAV vectors.
-Regulatory Momentum: FDA guidance and accelerated approvals streamline paths from trials to market, spurring GMP-compliant production.
-Market Hurdles: Elevated manufacturing expenses, immunogenicity risks, and stringent purity regulations pose key scalability challenges.
Regional Insights:
-North America: 49.82% (Largest share, driven by advanced biotech firms, research institutions, and high R&D investments in gene therapies).
-Asia Pacific: 25% (Fastest growing, fueled by rapid biotech expansion, government incentives, and rising clinical trials in China, India, and Japan).
-Europe: 15% (Supported by established pharma collaborations and regulatory frameworks in Germany, UK, and France).
-Latin America: 5% (Emerging potential through increasing biologics infrastructure and partnerships).
-Middle East & Africa: 4.18% (Moderate growth from healthcare investments and gene therapy adoption).
Market Opportunities & Challenges: Adeno Associated Virus Vectors Manufacturing Market 2026
-Opportunities: A "Scalable Platform Surge" accelerates via biopharma-CDMO partnerships for suspension-based bioreactors and helper-free systems, easing clinical-to-commercial transitions. Capsid engineering for tissue-specific, immune-evasive serotypes, alongside AI-driven process optimization, unlocks precision medicine in oncology and neurology; emerging Asia-Pacific hubs with GMP expansions de-risk supply for rare disease pipelines.
-Challenges: Persistent "Empty Capsid Impurity" burdens demand costly purification innovations, while bioreactor yield variability hampers high-titer scalability amid surging FDA approvals straining legacy infrastructure. Regulatory harmonization gaps across EMA/FDA/PRIME pathways complicate global filings, forcing CDMOs to navigate fragmented cold-chain logistics.
-Strategic Verdict: Next-gen AAV serotypes and automated multi-suite facilities propel 2026 dominance for agile manufacturers targeting diversified therapeutic pipelines.
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/adeno-associated-virus-vectors-manufacturing-market?ram
Market Segmentation Analysis:
-By Method: In Vitro Leads with Scalable Production
In vitro manufacturing dominates at 70% market share in 2025, leveraging cell culture systems for high-yield, scalable AAV vector production in controlled bioreactors.
In vivo methods hold 30%, used for direct viral delivery in preclinical models but limited by lower efficiency and regulatory hurdles for commercial scale.
-By Application: Gene Therapy Commands Majority Demand
Gene therapy captures 60% share, powering treatments for rare genetic diseases via precise AAV-mediated gene insertion.
Cell therapy follows at 20%, enhancing ex vivo cell modification; vaccine production at 15% for infectious disease immunization; others at 5% for oncolytics and diagnostics.
-By Therapeutic Area: Genetic Disorders Hold Top Position
Genetic disorders lead with 45% share, targeting conditions like spinal muscular atrophy and hemophilia via AAV gene correction.
Infectious diseases take 20%; neurological disorders 15%; hematological 10%; ophthalmic 5%; others 5%.
-By End-User: Pharma Companies Drive Commercialization
Pharmaceutical companies dominate at 50% share, investing heavily in AAV for late-stage gene therapies.
Biotechnology companies follow at 30%; academic/research institutes at 15%; others at 5%.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription?ram
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTW
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Adeno Associated Virus Vectors Manufacturing Market Set for Explosive Growth to US$ 9.98 Billion by 2034, Led by North America's 49.82% Market Share here
News-ID: 4392293 • Views: …
More Releases from DataM intelligence 4 Market Research LLP

United States LNG Storage Tank Market (2026): Generates USD $6.7Bn by 2030., Nor …
Market Size and Growth
LNG Storage Tank Market reached USD 4.5 billion in 2022 and is expected to reach USD 6.7 billion by 2030, growing with a CAGR of 5.5% during the forecast period 2024-2031.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://datamintelligence.com/download-sample/lng-storage-tank-market?kb
liquefied natural gas (LNG) in a nutshell and at the heart of this global energy dance are LNG storage tanks, the massive,…
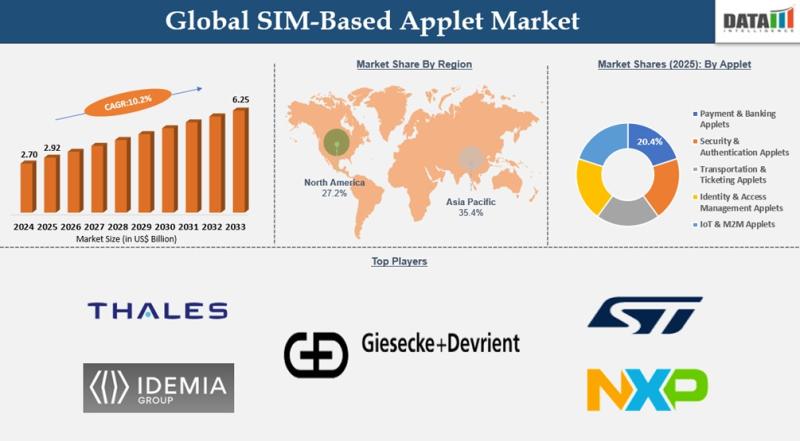
SIM Based Applet Market to Reach USD 6.25 Billion by 2033 at 10.2% CAGR | Asia P …
The SIM Based Applet Market reached USD 2.92 billion in 2025 and is projected to grow to USD 6.25 billion by 2033, expanding at a CAGR of 10.2% during the forecast period from 2026 to 2033. Market expansion is being driven by the rapid global adoption of mobile payments, increasing penetration of connected devices across smart homes and vehicles, and rising demand for secure digital transaction environments. As consumers and…
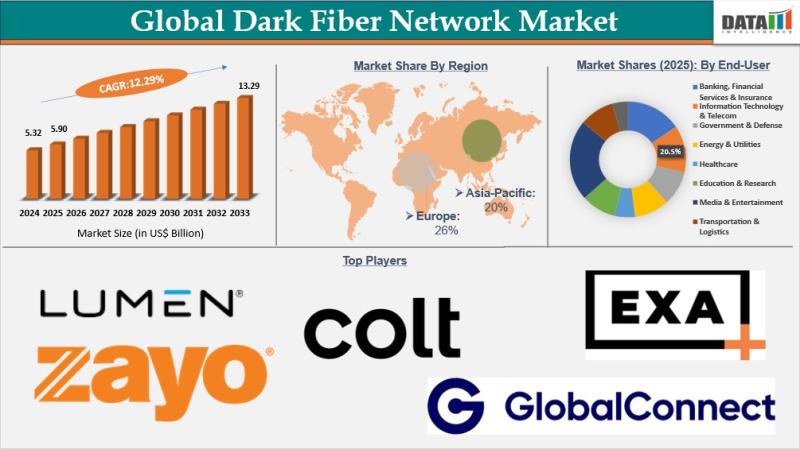
Dark Fiber Network Market to Reach USD 13.29 Billion by 2033 at 12.29% CAGR | No …
The Dark Fiber Network Market reached USD 5.32 billion in 2025 and is projected to grow to USD 13.29 billion by 2033, expanding at a CAGR of 12.29% during the forecast period from 2026 to 2033. Market growth is being propelled by the rapid expansion of hyperscale data centers, increasing reliance on cloud computing, and large scale deployment of 5G infrastructure. Enterprises and telecom operators are increasingly leasing dark fiber…

Brazil Food Supplement Market to Generate US$ 8,512.69 million by 2027 | Forecas …
Market Size and Growth
Brazil Food Supplement Market reached US$ 6,542.69 million in 2023 and is expected to reach US$ 8,512.69 million by 2027, growing with a CAGR of 7.02% during the forecast period 2024-2027.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://datamintelligence.com/download-sample/brazil-food-supplement-market?kb
As health-conscious habits continue to rise among Brazilians in today's fast-paced lifestyle, the food supplement sector is not just expanding it's reshaping…
More Releases for AAV
AAV Gene Therapy: $5.72B to $39.45B | 21.3% CAGR
Why are AAV vectors considered one of the safest and most efficient gene delivery systems?
Adeno-associated virus (AAV) vectors have gained prominence as one of the most reliable, safe, and clinically effective viral delivery platforms in the gene therapy landscape. Their favorable safety profile, ability to deliver therapeutic genes with precision, and long-term expression capabilities make them ideal for addressing rare diseases, inherited conditions, and chronic disorders.
One of the core reasons…
AAV Vector Transfection Kits Market Key Players, Share and Forecast Outlook
"The global market for AAV (Adeno-Associated Virus) vector transfection kits is poised for significant growth, currently valued at approximately $1.2 billion in 2024. This market is projected to reach around $3 billion by 2034, reflecting a robust compound annual growth rate (CAGR) of 9.5% during the forecast period of 2025-2034. "
Exactitude Consultancy., Ltd. released a research report titled "AAV Vector Transfection Kits Market". This report covers the global AAV Vector…
ProBio offers AAV One-stop Solution for AAV vector
AAV One-stop Solution
Process development for triple transfection
Support regulatory filing
AAV vector is widely used delivery vehicle due to its high safety and effectiveness in delivering Gene of Interest (GOI). ProBio is broadening its business in AAV services [https://www.probiocdmo.com/gct-one-stop-aav.html]to cater to the market demand.
Image: https://www.probiocdmo.com/img/probio/gct-one-stop-aav-banner.jpg
One-stop Solution for AAV
ProBio offers services from cell banking, process development, AAV packaging [https://www.probiocdmo.com/gct-one-stop-aav.html], analytical development, to GMP manufacturing and stability test for AAV vector. ProBio is also…
AAV Contract Development And Manufacturing Organizations Market 2024 Insights an …
In recent years, the global AAV Contract Development And Manufacturing Organizations Market has witnessed a dynamic shift, influenced by changing consumer preferences, technological advancements, and a growing emphasis on sustainability. The Research report on AAV Contract Development And Manufacturing Organizations Market presents a complete judgment of the market through strategic insights on future trends, growth factors, supplier landscape, demand landscape, Y-o-Y growth rate, CAGR, pricing analysis. It also provides and…
Adeno-Associated Virus (AAV) Vectors in Gene Therapy Pipeline Outlook Report 202 …
DelveInsight has released its latest report titled "AAV Vectors in Gene Therapy Pipeline Insight 2024" offering extensive insights into over 70 companies and more than 235 pipeline drugs within the AAV vectors gene therapy landscape. This comprehensive report includes detailed profiles of pipeline drugs across clinical and nonclinical stages, alongside thorough assessments based on product type, development stage, route of administration, and molecule type. Additionally, it features an analysis of…
Adeno-Associated Virus (AAV) CDMO Services Market Opportunities and Forecast 202 …
Data Library Research newly added a research report on the Adeno-Associated Virus (AAV) CDMO Services Market, which represents a study for the period from 2022 to 2029. The research study provides a near look at the market scenario and dynamics impacting its growth. This report highlights the crucial developments along with other events happening in the market which are marking on the growth and opening doors for future growth in…