Press release
Electronic Clinical Outcome Assessment Solutions Market Set for Explosive Growth to US$ 7.93 Billion by 2033, Led by North America's 37% Market Share | Key Players - IQVIA Holdings Inc, Oracle Corporation, Dassault Systems SE
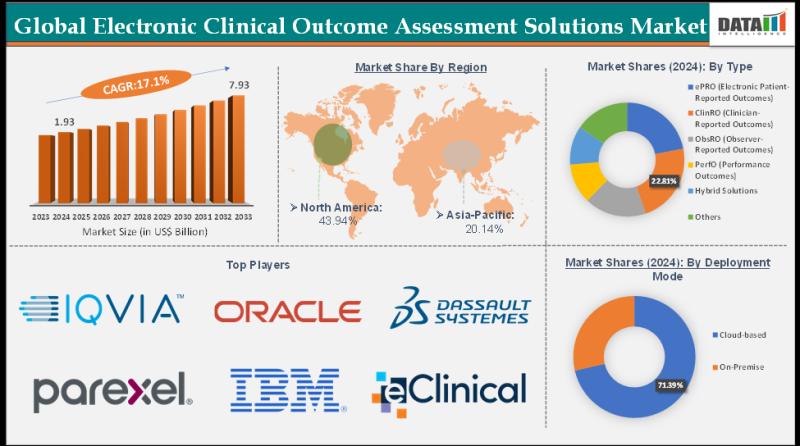
The Global Electronic Clinical Outcome Assessment Solutions Market size reached US$ 1.93 Billion in 2024 from US$ 1.67 Billion in 2023 and is expected to reach US$ 7.93 Billion by 2033, growing at a CAGR of 17.1% during the forecast period 2025-2033.Market growth is driven by the surging demand for patient-centric clinical trials, regulatory mandates for electronic data capture (e.g., FDA's eCOA guidelines), and the need for real-time, accurate patient-reported outcomes in drug development. Advancements in cloud-based eCOA platforms, integration with wearables and AI analytics, rising adoption in pharma and biotech R&D, and expanding decentralized trial models are further accelerating market expansion.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/electronic-clinical-outcome-assessment-solutions-market?ram
Key Industry Developments
United States:
✅ January 2026: Medidata Solutions launched an advanced eCOA module integrated with AI-driven analytics for real-time patient data capture and predictive outcome modeling, enhancing clinical trial efficiency and accuracy in decentralized trials.
✅ December 2025: EDETEK released CONFORM eClinical Version 5.2, featuring blockchain-secured data integrity for eCOA endpoints in multi-site US trials.
✅ November 2025: Oracle Health introduced Cerner eCOA enhancements with voice-activated PRO collection and natural language processing for qualitative assessments.
Asia Pacific / Japan:
✅ January 2026: Signant Health Japan expanded its TrialKit eCOA platform with multilingual support for PROs in kanji and local dialects, targeting Japan's precision medicine initiatives.
✅ December 2025: Medable Japan unveiled a decentralized eCOA solution with 5G-enabled real-time syncing for performance outcomes in neurology trials.
✅ October 2025: Veeva Systems APAC launched Vault eCOA with edge computing for low-latency data capture in high-volume trials across China and India.
Strategic Mergers and Acquisitions:
✅ Clario solidified its leadership in digital endpoint solutions by completing the acquisition of WCG's electronic clinical outcome assessment (eCOA) business in May 2025, enhancing data collection and analysis capabilities for neuroscience drug development.
✅ Suvoda and Greenphire merged in January 2025 to create a unified clinical trial technology platform, integrating eCOA, randomization, supply management, eConsent, and patient payments for streamlined trial operations.
Key Players:
IQVIA Holdings Inc. | Oracle Corporation | Dassault Systèmes SE | Parexel International Corporation | IBM Corporation | Signant Health | Clario | eClinical Solutions LLC | Castor | Anju Software
Strategic Leadership Report: Top 5 Players in Electronic Clinical Outcome Assessment Solutions Market 2026
-IQVIA Holdings Inc: Launched the enhanced IQVIA eCOA platform with AI-driven real-time data monitoring and patient-centric features like offline reporting and automated compliance prompts, achieving up to 95% compliance rates while supporting diverse populations across global clinical trials.
-Oracle Corporation: Introduced Oracle Clinical One Electronic Clinical Outcomes Assessment module integrated with Cerner health records, enabling seamless BYOD data capture, caregiver inputs, and adaptive trial designs to accelerate regulatory-ready outcomes in decentralized studies.
-Dassault Systèmes SE: Expanded the Medidata Rave eCOA suite with cloud-based multimodal assessments including wearables integration and predictive analytics for PROs, PROMs, and ClinROs, improving data quality and patient adherence through intuitive mobile interfaces.
-Parexel International Corporation: Deployed Parexel Percept eCOA solution featuring real-time risk-based monitoring and multilingual BYOD provisioning, streamlining complex endpoint collection and mid-study amendments to enhance trial efficiency and patient engagement.
-Signant Health: Advanced the MyHealthPlayer eCOA platform with embedded AI analytics for anomaly detection in patient-reported outcomes and interoperability with EDC systems, delivering high-integrity data flows and customizable workflows for oncology and rare disease trials.
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=electronic-clinical-outcome-assessment-solutions-market?ram
Market Drivers and Key Trends:
-Decentralized Trials Boom: Shift to patient-centric, remote clinical trials demands real-time eCOA data capture via mobile apps and wearables for enhanced patient compliance and reduced site visits.
-Regulatory Mandates: FDA and EMA emphasis on patient-reported outcomes (PROs) in drug labeling drives adoption of validated eCOA platforms to ensure high-quality, standardized evidence generation.
-Cloud Technology Surge: Scalable cloud-based eCOA solutions enable seamless integration with EHRs, accelerating clinician-reported outcomes (ClinRO) and supporting a projected 16% CAGR through 2031.
-Data Accuracy Imperative: Rising complexity in healthcare R&D requires unified systems for precise, timely outcome assessments, minimizing errors in global trials.
-Market Challenges: High implementation costs, data privacy concerns under GDPR/HIPAA, and integration hurdles with legacy systems pose key barriers to widespread adoption.
Regional Insights:
-North America: 37% (Largest share, driven by advanced clinical infrastructure and FDA regulations).
-Asia Pacific: 25% (Fastest growing at 21.5% CAGR, fueled by R&D expansion in China, India, and Japan).
-Europe: 20% (Supported by regulatory frameworks and steady digital health investments).
Market Opportunities & Challenges: Electronic Clinical Outcome Assessment Solutions Market 2026
-Opportunities: A "Patient-Centric Digital Shift" accelerates decentralized trials; integration of AI-driven analytics and wearable sensors enables real-time PRO (Patient-Reported Outcomes) capture for chronic disease studies. FDA's eCOA guidance expansions and cloud-based interoperability standards offer streamlined compliance pathways for global pharma sponsors.
-Challenges: Legacy system interoperability gaps inflate integration costs, while stringent GDPR/HIPAA data privacy mandates complicate cross-border trial deployments. Success demands mastering vendor consolidation amid CRO consolidation waves.
-Strategic Verdict: High-adoption ePRO platforms and RWE (Real-World Evidence) fusion categories propel 2026 dominance for agile innovators.
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/electronic-clinical-outcome-assessment-solutions-market?ram
Market Segmentation Analysis:
-By Type of Solution: ePRO Leads with Patient-Centric Focus
ePRO holds the largest share at 45% in 2024, enabling direct patient input via apps for real-time symptoms in clinical trials.
ClinRO captures 25%, relying on clinician assessments for objective efficacy data.
ObsRO and PerfO split 15% and 10%, with ObsRO for caregiver observations in pediatrics and PerfO measuring functional tasks like mobility.
Hybrid Solutions and Others take 5%, blending formats for flexibility.
-By Deployment Mode: Cloud-Based Dominates for Scalability
Cloud-based solutions command 70% share, offering remote access, data integration, and cost savings for global trials.
On-Premise holds 30%, preferred by organizations needing data control and compliance in regulated environments.
-By End-User: Pharma & Biopharma Companies Lead Innovation
Pharmaceutical & Biopharmaceutical Companies dominate at 50%, driving trial efficiency and regulatory submissions.
CROs follow at 25%, outsourcing data capture for speed.
Academic & Research Institutions and Hospitals/Healthcare Providers take 15% and 8%, focusing on studies and patient care.
Others account for 2%.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription?ram
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTW
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Electronic Clinical Outcome Assessment Solutions Market Set for Explosive Growth to US$ 7.93 Billion by 2033, Led by North America's 37% Market Share | Key Players - IQVIA Holdings Inc, Oracle Corporation, Dassault Systems SE here
News-ID: 4391427 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
Transthyretin Amyloid Cardiomyopathy Market Set for Explosive Growth to US$ 64.0 …
Market Overview
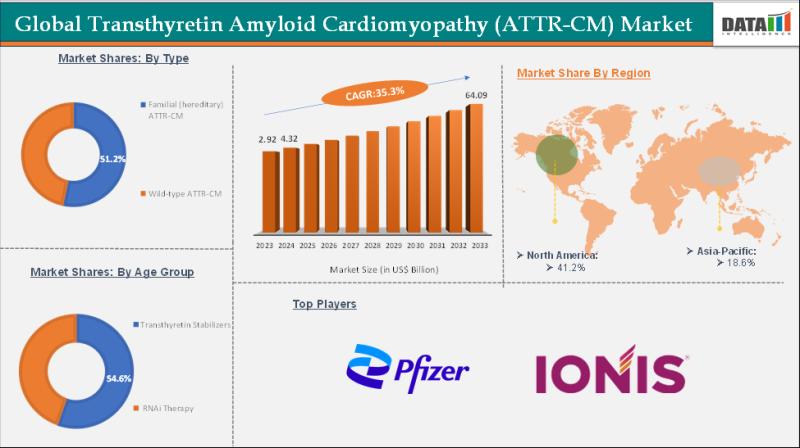
The Transthyretin amyloid cardiomyopathy (ATTR-CM) was valued at US$ 2.92 Billion n 2023. The global transthyretin amyloid cardiomyopathy (ATTR-CM) market size reached US$ 4.32 Billion in 2024 and is expected to reach US$ 64.09 Billion by 2033, growing at a CAGR of 35.3% during the forecast period 2025-2033.The market is growing due to rising disease awareness, improved diagnostic techniques, increasing elderly population, and advancements in targeted therapies. Regulatory approvals…

IoT and Virtual Hospital Market to Reach USD 68.40 Billion by 2033 at 16.8% CAGR …
The IoT and Virtual Hospital Market was valued at USD 20.12 billion in 2024 and is projected to reach USD 68.40 billion by 2033, expanding at a CAGR of 16.8% during the forecast period from 2025 to 2033. This rapid growth reflects the convergence of Internet of Things (IoT) technologies with virtual hospital platforms to deliver connected, accessible, and efficient healthcare services. Increasing patient demand for remote care, rising chronic…

Procurement as a Service Market Set for Explosive Growth to US$ 16.74 Billion by …
The Global Procurement as a Service Market reached US$6.30 billion in 2023, with a rise to US$6.87 billion in 2024, and is expected to reach US$16.74 billion by 2033, growing at a CAGR of 10.4% during the forecast period 2025-2033.
Market growth is driven by the rising adoption of digital procurement solutions and increasing demand for cost-efficient, scalable sourcing models across industries. Advancements in strategic sourcing, expanding needs for supplier management,…

Smart Retail Industry Market Forecast for Robust Growth at 7.7% CAGR During 2024 …
Market Overview
Retail Industry Market reached US$ 28,680.3 billion in 2023 and is expected to reach US$ 51,554.7 billion by 2031, growing with a CAGR of 7.7% during the forecast period 2024-2031.The market is growing due to rapid urbanization, rising disposable incomes, expansion of e-commerce platforms, omnichannel strategies, digital payment adoption, and changing consumer preferences. Technological advancements, personalized shopping experiences, and improved supply chain networks further accelerate market expansion globally.
Get a…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…