Press release
AAV Packaging Services Market Projected to Reach USD 3.8 Billion by 2036 as Gene Therapy Pipelines Mature at 11.2% CAGR
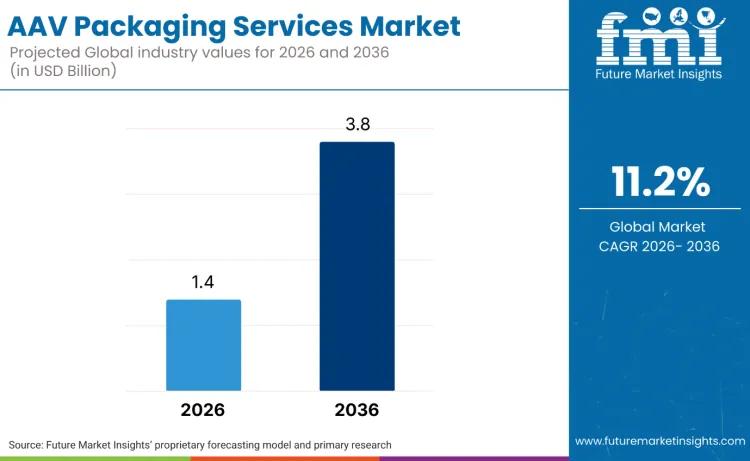
The global AAV packaging services market is poised for significant expansion, with its valuation expected to rise from USD 1.4 billion in 2026 to USD 3.8 billion by 2036. This steady growth, characterized by a compound annual growth rate (CAGR) of 11.2%, reflects the accelerating adoption of adeno-associated virus vectors in gene therapy. As clinical pipelines expand across rare genetic conditions and neuromuscular disorders, the industry is seeing a decisive shift toward specialized, GMP-compliant manufacturing platforms to meet rigorous regulatory standards.Read Full Report:https://www.futuremarketinsights.com/reports/aav-packaging-services-market
Direct Answers
Market size 2026? The AAV packaging services market is projected to reach USD 1.4 billion.
Market size 2035? While the 2035 value is on the growth path, the market is forecast to reach USD 3.8 billion by 2036.
CAGR? The market is expected to expand at a CAGR of 11.2% from 2026 to 2036.
Leading product segment(s) and shares? GMP Clinical AAV Packaging (Phase I/II) leads the service type segment with a 38.2% share. Regarding applications, Neuromuscular and CNS applications lead with 28.6% share. (Note: Baking and grilling & frying segments mentioned in the master prompt are not applicable to the AAV packaging source provided).
Leading material type and share? Not applicable to this source; however, GMP clinical packaging services dominate the service landscape with a 38.2% share.
Leading end use and share? Not applicable to this source; however, Neuromuscular and CNS applications account for 28.6% of the therapeutic application share.
Key growth regions? North America (USA), East Asia (China), South Asia (India), and Europe (Germany, France).
Top companies? Thermo Fisher Scientific Inc., Lonza Group AG, Catalent, Inc., Viralgen Vector Core S.L., WuXi Advanced Therapies Inc., Oxford Biomedica plc, FUJIFILM Diosynth Biotechnologies, Andelyn Biosciences, AGC Biologics, and Batavia Biosciences.
Market Momentum (YoY Path)
The AAV packaging services market is entering a decade of sustained capital infusion and infrastructure scaling. Starting at a valuation of USD 1.4 billion in 2026, the sector is expected to witness steady year-over-year gains as biotechnology firms transition programs from research-grade to commercial supply. The momentum is projected to carry the market through intermediate milestones in 2028 and 2030, with heightened activity in 2031 and 2033 as late-stage clinical trials conclude. By 2035, the market will be positioned just below its 2036 forecast of USD 3.8 billion, driven by the commercialization of approved AAV-based therapies and the saturation of automated production systems.
Why the Market is Growing
The primary catalyst for the AAV packaging services market is the rising demand for gene therapies, which has forced a surge in outsourcing to CDMOs capable of delivering scalable, compliant vectors. As the complexity of AAV capsid modifications and payload optimization increases, the technical barriers to in-house manufacturing have risen. Consequently, pharmaceutical organizations are prioritizing safety, traceability, and time-to-clinic efficiency, driving investment into integrated solutions that combine upstream production with high-yield analytics.
Segment Spotlight
Service Type:GMP clinical packaging services command the largest market share at 38.2%. This dominance is fueled by the high volume of gene therapies currently in Phase I and Phase II trials, necessitating batch release testing and regulatory documentation for investigational new drug applications.
Therapeutic Application:Neuromuscular and CNS applications represent 28.6% of the market. This is due to the ability of AAV vectors to cross the blood-brain barrier, though it requires sophisticated vector engineering and high viral doses for efficient gene transfer.
End Use:Emerging biotech and CGT start-ups are significant drivers, as they often lack the capital for internal manufacturing. This leads to a heavy reliance on specialized contract manufacturing organizations to navigate the transition from research-grade to commercial-grade production.
Drivers, Opportunities, Trends, Challenges
Demand is driven by a fundamental shift toward the commercialization of AAV-based therapies. As more programs move through the pipeline, there is a sustained need for platforms that can support market supply requirements while maintaining vector potency. The industry is currently moving away from traditional adherent cell culture toward scalable suspension platforms, which offer improved manufacturing economics and higher yields.
Opportunities exist in the integration of artificial intelligence and automated production systems. These technologies allow for precise control over vector quality, reducing batch-to-batch variability. Furthermore, the rise of "pure-play" CDMOs provides a specialized alternative for developers who require end-to-end solutions, from vector design consultation to regulatory support across multiple jurisdictions.
Trends indicate a clear transition toward integrated GMP manufacturing platforms that support the full development lifecycle. Companies are increasingly investing in proprietary platforms, such as suspension-based HEK293 systems, to boost productivity. Platform standardization and the implementation of single-use manufacturing systems are also becoming standard to eliminate cross-contamination risks.
Challenges remain in the technical complexity of AAV manufacturing. Maintaining vector stability and minimizing degradation during the packaging process are vital for clinical success. Additionally, navigating the evolving regulatory landscape across different regions requires deep expertise, making the choice of a manufacturing partner a critical strategic decision for gene therapy developers.
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates: https://www.futuremarketinsights.com/reports/brochure/rep-gb-31823
Request for Sample Report | Customize Report |purchase Full Report -https://www.futuremarketinsights.com/reports/sample/rep-gb-31823
Competitive Landscape
The competitive environment for the AAV packaging services market is characterized by stiff competition among established CDMOs like Thermo Fisher Scientific, Lonza, and Catalent. These giants are competing on manufacturing capacity and the integration of cutting-edge technologies like AI and automation. Strategic acquisitions are common, such as Lonza's acquisition of Genentech's Vacaville site and Oxford Biomedica's acquisition of ABL Europe. While North American players lead in infrastructure, European providers are focusing on regulatory compliance, and Asia-Pacific firms are leveraging cost advantages and high-throughput systems to gain ground.
Scope of the Report
Quantitative Units: Revenue in USD billion.
Segmentation: Service Type (Research-grade, GMP Clinical, Commercial, Analytical), Therapeutic Application (Ophthalmology, Neuromuscular, Oncology, etc.), and End User (Biotech, Pharma, Academic, CROs).
Regions: North America, Europe, East Asia, South Asia, Latin America, Middle East & Africa.
Countries: USA, China, India, Germany, France, and 40+ others.
Key Companies: Thermo Fisher Scientific, Lonza, Catalent, Viralgen, WuXi Advanced Therapies, Oxford Biomedica, and others.
Explore More Related Studies Published by FMI Research:
Digital Colony Counter Market: https://www.futuremarketinsights.com/reports/digital-colony-counter-market
Baby Breathing Monitor Market: https://www.futuremarketinsights.com/reports/baby-breathing-monitor-market
Gastrointestinal Therapeutics Market: https://www.futuremarketinsights.com/reports/gastrointestinal-therapeutics-market
Preterm Birth Prevention and Management Market: https://www.futuremarketinsights.com/reports/preterm-birth-prevention-and-management-market
Infant Gut Health Market: https://www.futuremarketinsights.com/reports/infant-gut-health-market
Why FMI: Decisions that Change Outcomes- https://www.futuremarketinsights.com/why-fmi
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us - sales@futuremarketinsights.com
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-347-918-3531
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release AAV Packaging Services Market Projected to Reach USD 3.8 Billion by 2036 as Gene Therapy Pipelines Mature at 11.2% CAGR here
News-ID: 4389978 • Views: …
More Releases from Future Market Insights
Cryo Peptides Market Projected to Reach USD 7.3 Billion by 2036, Driven by Proto …
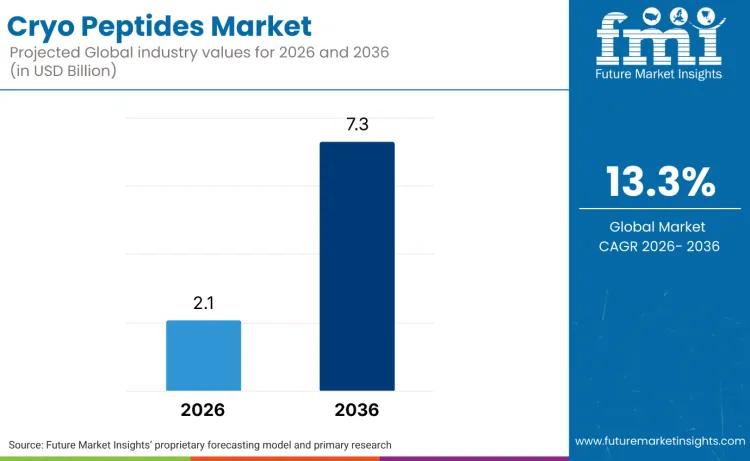
The global Cryo Peptides Market is witnessing a structural shift as dermocosmetic platforms consolidate capital around science-led actives and physician-adjacent distribution. According to the latest market analysis by Future Market Insights (FMI), the sector is valued at USD 2.1 billion in 2026 and is projected to surge to USD 7.3 billion by 2036, expanding at a robust 13.3% CAGR.
This expansion is increasingly defined by "protocol-led demand," where high-efficacy skincare is…
BioSignal Serums Market to Surpass USD 11.4 Billion by 2036 as Precision Biomark …
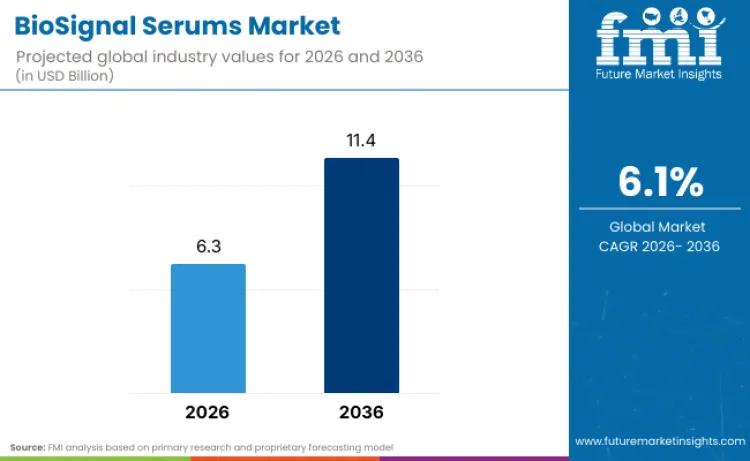
The global BioSignal Serums Market is undergoing a fundamental transition from traditional surface-level cosmetics to evidence-based regenerative science. According to the latest market analysis by Future Market Insights (FMI), the industry is projected to reach a valuation of USD 6.3 billion in 2026. Maintaining a steady compound annual growth rate (CAGR) of 6.1%, the market is expected to scale to USD 11.4 billion by 2036.
This evolution is fueled by a…
Global Drop Shape Analyzer Market Set to Reach USD 805.6 Million by 2036, Driven …
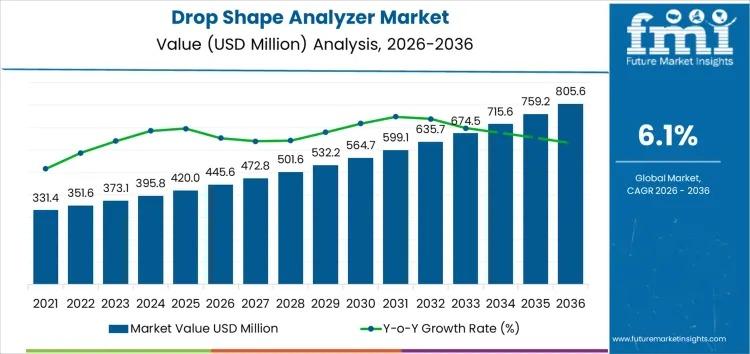
The Drop Shape Analyzer Market is witnessing robust growth, projected to expand from USD 445.6 million in 2026 to USD 805.6 million by 2036 at a CAGR of 6.1%. Driven by rising demand across coatings, electronics, medical devices, and academic research, the market is increasingly essential for industries that rely on precise surface and interfacial property measurements. Contact angle measurement leads the market with a 40% share, reflecting its significance…
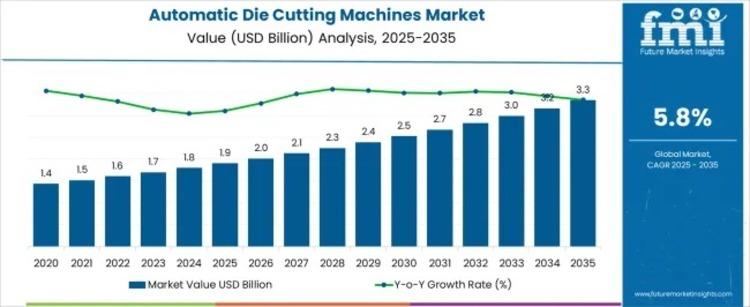
Global Automatic Die Cutting Machines Market 2025-2035: Automation & Efficiency …
Market Overview
The Automatic Die Cutting Machines Market is experiencing steady growth as industries seek automation, precision, and efficiency in production processes. Valued at USD 1.9 billion in 2025, the market is expected to reach USD 3.3 billion by 2035, growing at a CAGR of 5.8%. Key drivers include the demand for high-speed, error-free cutting, especially in automotive, packaging, and paper industries where quality and turnaround time are critical.
Automation continues to…
More Releases for AAV
AAV Gene Therapy: $5.72B to $39.45B | 21.3% CAGR
Why are AAV vectors considered one of the safest and most efficient gene delivery systems?
Adeno-associated virus (AAV) vectors have gained prominence as one of the most reliable, safe, and clinically effective viral delivery platforms in the gene therapy landscape. Their favorable safety profile, ability to deliver therapeutic genes with precision, and long-term expression capabilities make them ideal for addressing rare diseases, inherited conditions, and chronic disorders.
One of the core reasons…
AAV Vector Transfection Kits Market Key Players, Share and Forecast Outlook
"The global market for AAV (Adeno-Associated Virus) vector transfection kits is poised for significant growth, currently valued at approximately $1.2 billion in 2024. This market is projected to reach around $3 billion by 2034, reflecting a robust compound annual growth rate (CAGR) of 9.5% during the forecast period of 2025-2034. "
Exactitude Consultancy., Ltd. released a research report titled "AAV Vector Transfection Kits Market". This report covers the global AAV Vector…
ProBio offers AAV One-stop Solution for AAV vector
AAV One-stop Solution
Process development for triple transfection
Support regulatory filing
AAV vector is widely used delivery vehicle due to its high safety and effectiveness in delivering Gene of Interest (GOI). ProBio is broadening its business in AAV services [https://www.probiocdmo.com/gct-one-stop-aav.html]to cater to the market demand.
Image: https://www.probiocdmo.com/img/probio/gct-one-stop-aav-banner.jpg
One-stop Solution for AAV
ProBio offers services from cell banking, process development, AAV packaging [https://www.probiocdmo.com/gct-one-stop-aav.html], analytical development, to GMP manufacturing and stability test for AAV vector. ProBio is also…
AAV Contract Development And Manufacturing Organizations Market 2024 Insights an …
In recent years, the global AAV Contract Development And Manufacturing Organizations Market has witnessed a dynamic shift, influenced by changing consumer preferences, technological advancements, and a growing emphasis on sustainability. The Research report on AAV Contract Development And Manufacturing Organizations Market presents a complete judgment of the market through strategic insights on future trends, growth factors, supplier landscape, demand landscape, Y-o-Y growth rate, CAGR, pricing analysis. It also provides and…
Adeno-Associated Virus (AAV) Vectors in Gene Therapy Pipeline Outlook Report 202 …
DelveInsight has released its latest report titled "AAV Vectors in Gene Therapy Pipeline Insight 2024" offering extensive insights into over 70 companies and more than 235 pipeline drugs within the AAV vectors gene therapy landscape. This comprehensive report includes detailed profiles of pipeline drugs across clinical and nonclinical stages, alongside thorough assessments based on product type, development stage, route of administration, and molecule type. Additionally, it features an analysis of…
Adeno-Associated Virus (AAV) CDMO Services Market Opportunities and Forecast 202 …
Data Library Research newly added a research report on the Adeno-Associated Virus (AAV) CDMO Services Market, which represents a study for the period from 2022 to 2029. The research study provides a near look at the market scenario and dynamics impacting its growth. This report highlights the crucial developments along with other events happening in the market which are marking on the growth and opening doors for future growth in…