Press release
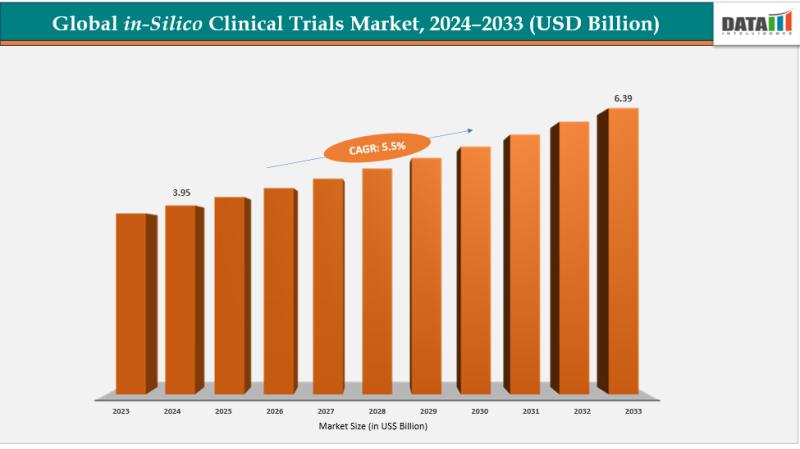
In-Silico Clinical Trials Market Size is expected to reach US$ 6.39 Billion with a CAGR of 5.5% by 2033, Dominate by North America with 44% Market Share
The global In-Silico clinical trials market size reached US$ 3.76 Billion in 2023 with a rise of US$ 3.95 Billion in 2024 and is expected to reach US$ 6.39 Billion by 2033, growing at a CAGR of 5.5% during the forecast period 2025-2033.The In-Silico Clinical Trials Market is growing due to advanced computational models, reduced R&D costs, faster drug development, improved patient safety, and rising adoption of AI-driven simulations in pharmaceutical research.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/in-silico-clinical-trials-market?prtk
United States: Key Industry Developments
✅ February 2026: Major pharmaceutical and biotech companies increasingly reported operational gains using AI‐assisted tools to speed clinical trial processes notably reducing site selection time and regulatory documentation effort, signaling expanded adoption of virtual trial support technologies throughout the U.S. drug development ecosystem.
✅ November 2025: Market analyses show the In‐Silico Clinical Trials Market expanding strongly in North America, with the U.S. accounting for a significant portion of global market share and adoption driven by AI‐modeling and digital twin techniques integrated into drug and device development workflows.
Notable Platform/Product Launches (2025)
✅ June 2025: Certara, Inc. released Phoenix version 8.6, an upgraded pharmacokinetic/pharmacodynamic (PK/PD) and toxicokinetic modeling platform enhancing computational efficiency and analytical power for in‐silico simulations now widely used in regulatory and clinical decision support.
Japan: Key Industry Developments
✅ January 2026: Japan's in silico clinical trials sector continues growth with increasing regulatory support for computational evidence and hybrid trial designs; adoption of virtual patient simulations and digital modeling tools has expanded, reinforcing the country's simulation‐based research infrastructure.
✅ November 2025: Regional analysis indicates that Japan's in silico clinical trials market is growing steadily, with rising application in medical device simulation and drug modeling, supported by broader AI and digital health initiatives.
Notable Platform/Product Launches (2025)
✅ June 2025: InSilicoTrials, a leading digital simulation platform developer, was accepted into the Microsoft for Startups Pegasus Program, expanding its cloud‐based simulation tools (including AI‐driven digital twin and virtual patient models) through Microsoft Azure infrastructure - a key platform scaling development in both global and Japanese markets.
In-Silico clinical trials Market Recent M&A activities:-
→ In January 2026, Dassault Systèmes acquired MedraAI, a startup specializing in AI-driven patient recruitment and synthetic control arms, for $410 million. This acquisition integrates MedraAI's machine learning algorithms into the 3DEXPERIENCE platform, specifically enhancing the "Living Heart" and "Living Brain" projects to allow for more accurate virtual patient modeling in neurology and cardiology trials.
→ In November 2025, Certara acquired InSilico Biome, a computational biology firm focused on gut microbiome modeling, for $125 million. The deal allows Certara to expand its Biosimulation software suite, providing pharmaceutical clients with the ability to simulate how oral drugs interact with the human microbiome before entering Phase I trials.
→ In October 2025, Simulations Plus acquired Quantitative Life Sciences (QLS), a consulting firm focused on Model-Informed Drug Development (MIDD), in a transaction valued at $38.5 million. This move was designed to consolidate expertise in mechanistic modeling and strengthen Simulations Plus's ability to provide regulatory-grade in-silico data for FDA and EMA submissions.
In-Silico clinical trials Market key Players:-
Certara, Dassault Systèmes, InSilicoTrials Technologies, Simulations Plus, VeriSIM Life, Physiomics Plc, ANSYS Inc., and Insilico Medicine, among others.
Top 5 Key Players Analysis:-
1. Certara
Leads with around 22% market share, leveraging comprehensive biosimulation and regulatory‐grade modeling platforms widely adopted in pharma and biotech.
Core strength lies in advanced PBPK, PK/PD simulation tools and deep regulatory engagement.
2. Dassault Systèmes
Holds approximately 18% share with strong systems biology and organ‐level digital twin modeling through BIOVIA and SIMULIA platforms.
Core strength is in multi‐scale simulation technologies integrated across drug discovery and device workflows.
3. Simulations Plus
Captures about 14.6% market share by providing robust PK/PD and PBPK modeling software trusted by regulators.
Core strength is in mechanistic absorption, distribution, and toxicity prediction tools across therapeutic development.
4. InSilicoTrials Technologies
Has roughly 11.3% share with cloud‐based in‐silico trial platforms and virtual patient modeling services.
Core strength lies in scalable virtual trial lifecyle tools that accelerate development timelines and reduce costs.
5. VeriSIM Life
Accounts for 9.5% share focused on quantitative systems pharmacology and virtual organ simulations.
Core strength is in precision simulation solutions supporting decision‐making in complex disease research.
In-Silico clinical trials Market Top Technological Partnerships (2026 & 2025):-
February 2026: Insilico Medicine expanded collaboration with Eli Lilly, transitioning from customer to strategic partner for AI-driven in silico trials simulating Phase II oncology endpoints with 85% accuracy.
December 2025: Simulations Plus partnered with FDA's INsilico Initiative, providing PK/PD modeling software for virtual bioequivalence studies reducing Phase I trials by 40% across 15 sponsors.
November 2025: Novadiscovery collaborated with Servier on digital twins for heart failure trials, generating 10,000 virtual patients to optimize diuretic dosing protocols pre-clinical start.
October 2025: Schrödinger teamed with Roche for quantum-accelerated protein dynamics simulations, predicting adverse events 72 hours earlier than traditional methods in CNS drug candidates.
September 2025: Virtonomy Health partnered with Medtronic on in silico pacemaker trials, modeling 5,000 implant scenarios to accelerate FDA 510(k) clearance by 6 months.
Buy Now & Unlock 360° Market Intelligence:https://www.datamintelligence.com/buy-now-page?report=in-silico-clinical-trials-market?prtk
In-Silico clinical trials Market Drivers :-
Pharmaceutical R&D faces mounting financial and time pressures with traditional drug development costs now often exceeding USD 2.6 billion per approved drug and development timelines stretching well over a decade. In‐silico clinical trials are emerging as a practical solution to both challenges.
By enabling virtual patient populations and predictive modeling early in the process, sponsors can optimize trial design, reduce costly late‐stage failures, and cut development costs by an estimated 30-50% compared with conventional methodologies. These efficiency gains not only reduce reliance on large cohorts but also accelerate the transition from concept to clinical evaluation.
Technological advances in artificial intelligence (AI), machine learning (ML), and high‐performance computing (HPC) are transforming in‐silico capabilities. These tools enhance model accuracy and predictive fidelity across pharmacokinetics, toxicology, and virtual cohort responses, enabling more reliable simulations of complex biological systems. The result? Faster iteration cycles, adaptable trial designs, and the ability to test hundreds of scenarios in parallel, driving broader adoption across pharmaceutical and medtech pipelines.
Regulatory bodies across major markets are increasingly recognizing computational evidence as a legitimate component of drug and device evaluation. Agencies such as the FDA and EMA now encourage model‐informed drug development (MIDD) approaches and pathways that integrate in‐silico data into submissions. This shift enhances confidence among sponsors and CROs, directly boosting adoption rates and shortening approval preparation timelines.
In-Silico clinical trials Market Regional Insights:-
North America
Market Share: 44 % of the global in‐silico clinical trials market, making it the largest regional segment due to strong R&D investment and early regulatory acceptance (e.g., FDA initiatives).
Insight: Northwestern dominance is supported by advanced healthcare infrastructure, high adoption of computational modeling, and concentration of leading pharma and biotech players.
2. Europe
Market Share: 28 % of global market share, placing it as the second‐largest region with strong clinical research frameworks and cross‐border regulatory support.
Insight: Growth is reinforced by public‐private collaborations and digital health initiatives across EU nations, although slower than North America.
3. Asia Pacific
Market Share: 20 % of the global market, fastest‐growing among major regions due to rising digital health uptake and expanding pharmaceutical R&D activity.
Insight: Adoption is accelerating in China, Japan, India, and Southeast Asia as healthcare digitization and AI‐enabled clinical modeling expand.
Get Customization in the report as per your requirements:https://www.datamintelligence.com/customize/in-silico-clinical-trials-market?prtk
In-Silico clinical trials Market Market Segmentation
The In-Silico Clinical Trials Market is segmented based on application and end user to provide a clear understanding of its diverse use cases and target audience. In terms of application, the market caters to various stages of healthcare and research processes. It supports drug development by simulating clinical trials to predict drug efficacy and safety, aids medical device evaluation through virtual testing and performance analysis, facilitates regulatory submissions by providing computational evidence to authorities, and enhances post-market surveillance by monitoring real-world outcomes. Additionally, it serves other applications such as personalized treatment planning and disease modeling.
Based on end users, the market targets organizations involved in healthcare innovation and research. Pharmaceutical and biotech companies leverage in-silico trials to accelerate drug discovery and reduce costs, while medical device manufacturers use it for design optimization and safety testing. Academic and research institutes adopt these simulations for experimental studies and predictive modeling, and contract research organizations (CROs) integrate them to enhance service offerings. The segment also includes other end users like hospitals and government research bodies that benefit from virtual trials for clinical and operational efficiency.
This research report delivers actionable insights, data-driven analysis, and future-ready perspectives that enable informed decision-making, reduce market risks, and uncover growth opportunities across the industry
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release In-Silico Clinical Trials Market Size is expected to reach US$ 6.39 Billion with a CAGR of 5.5% by 2033, Dominate by North America with 44% Market Share here
News-ID: 4384256 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
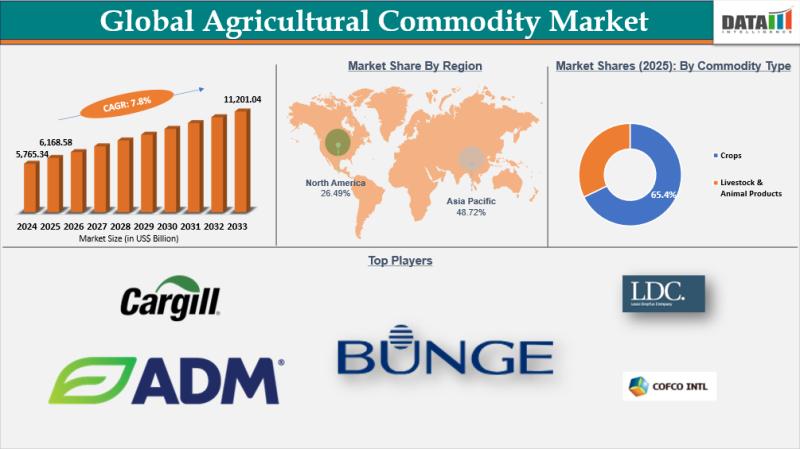
United States Agricultural Commodity Market to Grow US$ 11,201.04 billion by 203 …
Market Size and Growth
agricultural commodity market reached US$ 5,765.34 billion in 2024, rising to US$ 6,168.58 billion in 2025 and is expected to reach US$ 11,201.04 billion by 2033, growing at a strong CAGR of 7.8% during the forecast period from 2026 to 2033.
In 2025, the Asia-Pacific region led the global agricultural commodity market, capturing a 48.72% revenue share, fueled by its large population, robust food demand, extensive agricultural infrastructure,…

Astaxanthin Market to Grow US$ 3.47 Billion by 2033 | Growth Drivers, Trends & M …
Market Size and Growth
astaxanthin market was valued at US$ 1.61 Billion. The global Astaxanthin market size reached US$ 1.73 Billion in 2024 and is expected to reach US$ 3.47 Billion by 2033, growing at a CAGR of 8.1% during the forecast period 2025-2033.
Download Free Sample PDF Report (Get Higher Priority for Corporate Email ID):- https://datamintelligence.com/download-sample/astaxanthin-market?kb
astaxanthin a powerhouse antioxidant that's not just a pretty face in nature but a rising star…
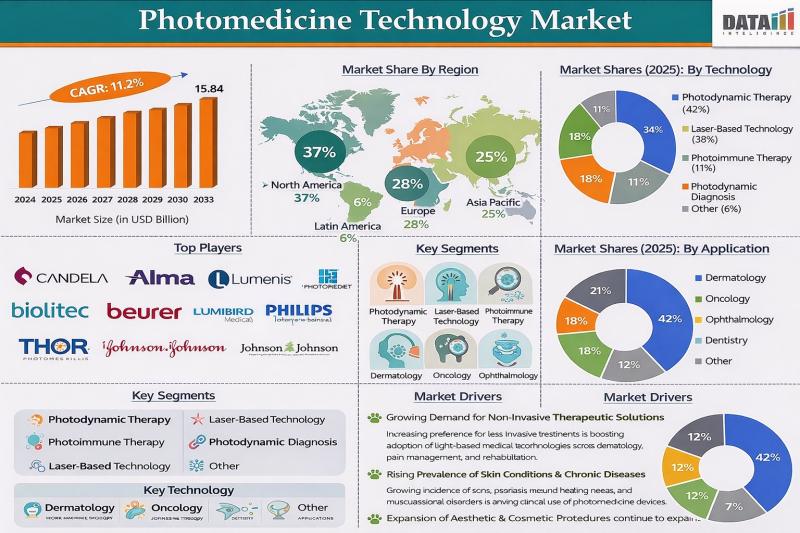
Photomedicine Technology Market to Reach USD 15.84 Billion by 2033 at 11.2% CAGR …
The Photomedicine Technology Market was valued at USD 6.25 billion in 2024 and is projected to reach USD 15.84 billion by 2033, expanding at a CAGR of 11.2% during the forecast period from 2025 to 2033. This rapid growth is driven by rising demand for non-invasive diagnostic and therapeutic solutions across dermatology, oncology, ophthalmology, and pain management. Photomedicine technologies, such as low-level laser therapy, photodynamic therapy, intense pulsed light systems,…

United States Pest Control Market Trends 2026 | IPM, Biocontrol, and Chemical So …
Market Size and Growth
Pest Control Market is expected to grow at a CAGR of 4.5% during the forecasting period (2024-2031).
Download Free Sample PDF Report (Get Higher Priority for Corporate Email ID):- https://datamintelligence.com/download-sample/pest-control-market?kb
Pest control refers to the management and elimination of unwanted insects, rodents, and other pests in residential, commercial, and agricultural settings. Advanced methods combine chemical treatments, biological solutions, and integrated pest management (IPM) to ensure safety, efficiency, and…
More Releases for Trials
Clinical Trials Management System Market Trends: How Decentralized Clinical Tria …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical Device Firms, CROs)- Market Outlook And Industry Analysis 2031"
The global Clinical Trials Management System market is estimated to reach…
Brazil Clinical Trials Market ANVISA Brazil Guidelines Brazil Clinical Trials Re …
Brazil Cancer Drugs Clinical Trials Insight 2024 Report Offering:
• Brazil Clinical Trials Market Opportunity 2024 and 2030 (In US$ Billion)
• Clinical Trials Regulatory Framework In Brazil
• Total Number of Cancer Drugs In Clinical Trials In Brazil
• Total Number Of Cancer Drugs Approved In Brazil
• 400 Pages Clinical Trials Insight On All Cancer Drugs In Clinical Trials By Company, Indication and Phase
• 80 Pages Clinical Insight On All Cancer Drugs Approved in Market By Company and Indication
• Insight…
Clinical Trials Management System Market Clinical Trials Management System Marke …
InsightAce Analytic announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical Device Firms, CROs)- Market Outlook And Industry Analysis 2031.
The global Clinical Trials Management System market is estimated to reach over USD…
Revolutionizing Clinical Trials: The Evolution of eCOA, eSource, and the Clinica …
The global eCOA, eSource & clinical trials market is valued at US$ 48 billion in 2023 and is expected to reach a market size of US$ 104 billion by the end of 2033, expanding rapidly at a CAGR of 8% over the next ten years. Worldwide demand for eCOA (electronic clinical outcome assessment) solutions is predicted to rise at a CAGR of 8.2% over the forecast period.
In the dynamic landscape…
Virtual Clinical Trials Market - Bridging the Gap: Virtual Clinical Trials Revol …
Newark, New Castle, USA - The latest report from Growth Plus Reports analyzes the production, potential applications, demand, major manufacturers, and SWOT analysis of the global Virtual Clinical Trials Market.
Virtual Clinical Trials Market: https://www.growthplusreports.com/report/virtual-clinical-trials-market/9106
The Virtual Clinical Trials Market Report assists in determining the optimum distribution methods for certain products as well as possible markets for future product launches. The report also analyses the purchase and supply trends that influence the…
Clinical Trials Market: Coronavirus Pandemic Pushes Sponsors, Patients to Adopt …
Clinical trials Market: Introduction
According to the report, the global clinical trials market was valued over US$ 46.7 Bn in 2019 and is projected to expand at a CAGR of ~5% from 2020 to 2030. High prevalence and increase in incidence rate of chronic diseases, and rise in R&D activities in biotechnology & pharmaceuticals industries are anticipated to drive the global clinical trials market from 2020 to 2030. North America is projected to…