Press release
United States Gene Therapy Market Expands from US$ 4.80 Billion to US$ 35.91 Billion by 2033 at 23.1% CAGR as North America Leads with 45% Share Driven by Novartis Roche and Pfizer
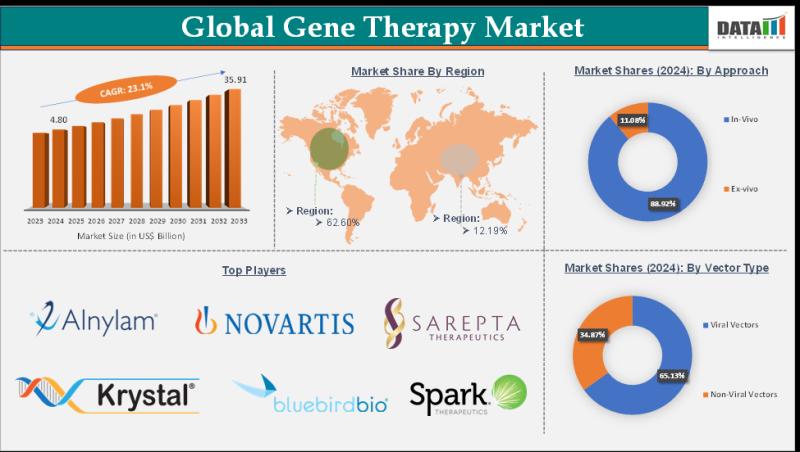
The Gene Therapy Market size reached US$ 4.80 Billion in 2024 and is expected to reach US$ 35.91 Billion by 2033, growing at a CAGR of 23.1% from 2025 to 2033., driven by rising demand for curative treatments for genetic disorders, rare diseases, and certain cancers, along with a strong clinical pipeline and increasing R&D investments.Growth is supported by increasing adoption across key applications such as oncology, blood disorders, neurological conditions, ophthalmology, and rare genetic diseases, propelled by continuous advancements in gene editing technologies (like CRISPR/Cas9), expanded regulatory approvals, and strategic collaborations between biotech and pharmaceutical companies. The development of more efficient viral and non-viral vector systems, coupled with a growing number of clinical trials and healthcare initiatives, enhances therapeutic precision, reducing long-term treatment burdens and improving patient outcomes globally. However, high therapy costs and complex regulatory frameworks continue to pose challenges to broader market penetration.
Download your exclusive sample report today (corporate email gets priority access):
https://www.datamintelligence.com/download-sample/gene-therapy-market?sindhuri
Gene Therapy Market: Competitive Intelligence
Novartis AG, Roche Holding AG, Spark Therapeutics (a Roche company), Pfizer Inc., Bristol-Myers Squibb Company, Gilead Sciences Inc. (including Kite Pharma and other subsidiaries), Regenxbio Inc., Sarepta Therapeutics Inc., BioMarin Pharmaceutical Inc., and others.
The Gene Therapy Market is strongly driven by leading players such as Novartis, Roche, Pfizer, Spark Therapeutics, and Bristol-Myers Squibb, who develop innovative gene-based therapeutic solutions for rare genetic disorders, oncology, hematology, neuromuscular diseases, and other unmet medical needs. Their cutting-edge vector technologies, delivery systems, and robust clinical pipelines enable targeted gene correction, replacement, or augmentation, offering transformative treatment options and improving patient outcomes. Increasing regulatory approvals, strategic collaborations, and rising investment in advanced biologics are fueling market expansion.
These companies' complementary strengths clinical-stage assets and commercial products from Novartis (e.g., Zolgensma), vector engineering and delivery expertise from Roche and Spark Therapeutics, diversified pipelines from Pfizer and Bristol-Myers Squibb, and focused innovation from specialty biotechs like Regenxbio and Sarepta-are enhancing competitive positioning. Strategic focus areas include viral and non-viral vector platform optimization, manufacturing scale-up, gene editing advancements (e.g., CRISPR/Cas systems), and partnerships aligned with personalized medicine and rare disease communities, strengthening competitiveness across therapeutic segments and global markets.
Get Customization in the report as per your requirements:
https://www.datamintelligence.com/customize/gene-therapy-market?sindhuri
Recent Key Developments - United States
✅ Nov 2025: Eli Lilly signed a major licensing deal with MeiraGTx valued at potentially over $475 million for rights to its experimental gene therapy for severe inherited vision loss (AAV-AIPL1), expanding Lilly's gene therapy portfolio in ophthalmology and gene-editing technologies.
✅ 2025: Verve Therapeutics acquisition by Eli Lilly (~$1.3 billion) drove strong investor response, as Lilly bolsters its gene-editing pipeline with VERVE-102 targeting atherosclerotic cardiovascular disease, reflecting strategic expansion into one-time genomic therapies.
✅ 2025 (Clinical & Safety): Sarepta Therapeutics faced regulatory scrutiny after the FDA requested a halt in shipments of its Duchenne muscular dystrophy gene therapy Elevidys following patient deaths, though the company continues offering treatment to younger patients amid safety review and operational adjustments.
Recent Key Developments - Global
✅ Oct 2025: AstraZeneca signed a deal (up to $555 million) with Algen Biotechnologies to develop gene therapies using Algen's AI-driven discovery platform targeting immune disorders, highlighting cross-industry efforts to accelerate gene therapy innovation.
✅ Dec 2025 / Jan 2026 (Regional): UAE approved the gene therapy Itvisma for spinal muscular atrophy (SMA) for patients aged two years and older, becoming the second country globally to authorize this advanced treatment, signaling broader international regulatory acceptance for transformative gene therapies.
✅ Global Partnerships & Pipeline Growth: Partnerships and co-development agreements (e.g., REGENXBIO collaborations on AAV-based retinal therapies) and pivotal trials like REGENXBIO's RGX-202 for Duchenne muscular dystrophy are progressing toward commercial submissions and expanded clinical impact.
✅ 1. M&A (Mergers & Acquisitions)
AbbVie acquires Capstan Therapeutics (H1 2025)
In June 2025, AbbVie Inc. agreed to acquire Capstan Therapeutics in a deal valued at up to $2.1 billion in upfront cash. Capstan's pipeline includes in vivo lipid nanoparticle platforms and CAR-T-based gene therapies for autoimmune diseases and other indications significantly expanding AbbVie's gene therapy and delivery capabilities.
Carlyle & SK Capital take Bluebird Bio private (Feb 2025)
In February 2025, Bluebird Bio was acquired and taken private by Carlyle Group and SK Capital strengthening private investment support for its gene therapy portfolio, which includes approved treatments such as Lyfgenia, Zynteglo, and Skysona.
✅ 2. New Product Launches & Regulatory Approvals
FDA approves Waskyra (etuvetidigene autotemcel) - Wiskott-Aldrich syndrome
In December 2025, the U.S. FDA approved Waskyra (etuvetidigene autotemcel) for the treatment of Wiskott-Aldrich syndrome (WAS) a rare, life-threatening genetic immune disorder granting orphan drug and regenerative medicine designations. It's notable as a gene therapy developed by a non-profit sponsor.
FDA approval expands Itvisma (Novartis) for Spinal Muscular Atrophy
In late 2025, the FDA approved Itvisma, a gene therapy extension of Zolgensma to treat spinal muscular atrophy (SMA) in patients age 2 and older, broadening the patient population for this high-impact gene-replacement therapy.
FDA ("Zevaskyn") as Second Gene Therapy for RDEB
Gene therapy prademagene zamikeracel (Zevaskyn) was approved in April 2025 as an autologous skin gene therapy for recessive dystrophic epidermolysis bullosa (RDEB), offering another advanced option for this rare skin disorder.
✅ 3. R&D Developments & Clinical Pipeline Progress
AskBio's AB-1009 IND Acceptance & Clinical Trial Launch
AskBio announced that the U.S. FDA accepted the IND application for AB-1009 a gene therapy for late-onset Pompe disease and initiated a Phase 1/2 PROGRESS-GT clinical trial, with first patient enrollment expected early 2026.
Solid Biosciences' SGT-212 for Friedreich's Ataxia
Solid Biosciences received FDA Orphan Drug designation for SGT-212 a dual-route AAV-based gene therapy for Friedreich's ataxia and recently dosed the first patient in its Phase 1b FALCON trial, marking an important clinical milestone.
REGENXBIO's Sura-vec & RGX-121 Progress
REGENXBIO highlighted key 2026 catalysts:
Surabgene lomparvovec (sura-vec), developed with AbbVie, could be the first gene therapy for a non-rare disease (wet AMD) with readouts expected in Q4 2026 and a Phase IIb/III expansion for diabetic retinopathy starting 1H 2026.
Regulatory planning and enrollment activities continue for clemidsogene lanparvovec (RGX-121) for MPS II (Hunter syndrome).
✅ 4. Technology & Market Advancements
Shift toward in vivo delivery & broader tissue targeting
Market outlooks note a shift from ex vivo to direct in vivo delivery routes using advanced vectors enabling gene therapies to reach deep anatomical sites like the central nervous system and treat previously inaccessible conditions.
Segments Covered in the Gene Therapy Market
By Approach
The market is segmented into in-vivo 60% and ex-vivo 40%, with in-vivo dominating due to its direct delivery of genetic material into patients, simplifying treatment workflows and reducing manufacturing complexity. In-vivo approaches are widely used in ophthalmology, neurology, and oncology applications. Ex-vivo gene therapy remains critical for blood disorders and rare diseases, particularly in CAR-T and stem cell-based therapies. Advancements in delivery systems and regulatory approvals continue to drive adoption across both approaches.
By Vector Type
Vector types include viral vectors 75% and non-viral vectors 25%, with viral vectors dominating due to their high transfection efficiency and long-term gene expression. Adeno-associated virus (AAV), lentivirus, and retrovirus are widely used across clinical and commercial gene therapies. Non-viral vectors are gaining traction due to improved safety profiles, lower immunogenicity, and reduced manufacturing costs. Ongoing R&D in lipid nanoparticles and polymer-based delivery systems supports future growth.
By Technique
Techniques comprise gene addition 45%, gene editing 35%, and gene silencing 20%, with gene addition leading due to its established clinical success and regulatory approvals for monogenic disorders. Gene editing is rapidly expanding with advancements in CRISPR-Cas, TALENs, and zinc finger nucleases, enabling precise and permanent genetic modifications. Gene silencing continues to grow in applications targeting overexpressed or harmful genes. Innovation in genome engineering technologies accelerates technique adoption.
By Application
Applications include rare diseases 35%, oncology 25%, blood disorders 15%, ophthalmology 10%, musculoskeletal conditions 8%, and others 7%, with rare diseases dominating due to high unmet medical needs and strong regulatory incentives such as orphan drug designations. Oncology applications are expanding rapidly with cell and gene-based immunotherapies. Blood disorders benefit from ex-vivo therapies for hemophilia and sickle cell disease. Ophthalmology remains a key area with approved retinal gene therapies.
Buy Now & Unlock 360° Market Intelligence:
https://www.datamintelligence.com/buy-now-page?report=gene-therapy-market?sindhuri
(Single User Report: USD 4350 & One Year Database Subscription: USD 12K)
Regional Analysis
North America - 45% Share
North America leads with 45% share driven by strong presence of biotechnology companies, advanced clinical trial infrastructure, and favorable regulatory pathways in the U.S. and Canada. Rare disease and oncology applications dominate. High R&D investments, venture funding, and early adoption of innovative gene therapies support regional leadership.
Europe - 25% Share
Europe accounts for 25% share supported by robust academic research, regulatory support for advanced therapy medicinal products (ATMPs), and growing commercialization across Germany, the UK, and France. Rare diseases and blood disorders are key application areas. Cross-border research collaborations and public funding initiatives drive market growth.
Asia Pacific - 20% Share
Asia Pacific holds 20% share driven by expanding biotech ecosystems, increasing clinical trials, and supportive government policies in China, Japan, and South Korea. Oncology and rare disease research are growing rapidly. Improving manufacturing capabilities and rising healthcare investments fuel regional expansion.
Request for 2 Days FREE Trial Access:
https://www.datamintelligence.com/reports-subscription?sindhuri
✅ Competitive Landscape
✅ Technology Roadmap Analysis
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Consumer Behavior & Demand Analysis
✅ Import-Export Data Monitoring
✅ Live Market & Pricing Trends
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release United States Gene Therapy Market Expands from US$ 4.80 Billion to US$ 35.91 Billion by 2033 at 23.1% CAGR as North America Leads with 45% Share Driven by Novartis Roche and Pfizer here
News-ID: 4382281 • Views: …
More Releases from DataM Intelligence 4Market Research LLP

Food Safety Testing Market to Reach US$40.1 Billion by 2029 at 9.0% CAGR, Led by …
The Food Safety Testing Market reached approximately US$ 24.5 billion in 2024 and is expected to grow to around US$ 40.1billion by 2029, expanding at a CAGR of about 9.0% from 2025 to 2029 as food industry stakeholders and regulatory bodies intensify efforts to ensure product safety, quality, and compliance across global supply chains.
Growth is supported by increasing demand across key segments such as microbiological testing, chemical residue analysis, allergen…

Food Ingredients Market to Reach US$538.68 Billion by 2032 at 5.45% CAGR, Driven …
The Food Ingredients Market Size reached US$ 352.34 billion in 2024 and is expected to reach US$ 538.68 billion by 2032, growing with a CAGR of 5.45% from 2025 to 2032. as food and beverage manufacturers intensify investments in value‐added and functional ingredients to meet evolving consumer preferences and regulatory standards.
Growth is supported by increasing demand across key categories such as flavors & fragrances, sweeteners, proteins & amino acids, emulsifiers,…

Aluminum Extrusion Market to Reach US$ 117.8 Billion by 2030 Driven by Infrastru …
The Aluminum Extrusion Market reached US$ 78.7 billion in 2022 and is expected to reach US$ 117.8 billion by 2030, growing at a CAGR of 5.7% during the forecast period 2025-2031.
Growth is driven by increasing demand from the construction, automotive, aerospace, and industrial sectors, where aluminum extrusions are widely used for their lightweight properties, strength, corrosion resistance, and design flexibility. Rising focus on fuel efficiency and vehicle lightweighting, along with…

Smart Farming Market to Reach US$22.45 Billion by 2031 at 9.1% CAGR, Led by Nort …
The Smart Farming Market reached US$ 11.12 billion in 2022 and is expected to reach US$ 22.45 billion by 2031, growing with a CAGR of 9.1% from 2024 to 2031. as agricultural stakeholders increasingly deploy IoT‐enabled technologies and data‐driven solutions to optimize farm productivity, resource efficiency, and sustainability.
Growth is supported by rising demand across key solutions such as precision agriculture tools, smart sensors, GPS‐enabled machinery, autonomous equipment, and farm management…
More Releases for Gene
DNA and Gene Cloning Services Market Expands with Growing Focus on Complex Gene …
InsightAce Analytic Pvt. Ltd. has announced the publication of a market research report titled "Global DNA and Gene Cloning Services Market by Type of Service Offered (Custom Cloning, Sub-cloning, Gene Synthesis, and Others), Type of Gene (Complex Gene, Standard Gene, and Others), End-User Industry (Pharmaceutical, Academic and R&D, and Biotechnology Companies, and Others)- Market Outlook and Industry Analysis 2034"
The DNA and Gene Cloning Services Market Size is valued…
Evolving Market Trends In The CRISPR Gene Editing Industry: Innovative Gene Ther …
The CRISPR Gene Editing Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Expected CRISPR Gene Editing Market Size During the Forecast Period?
The CRISPR gene editing market has grown exponentially in recent years. It will grow from $2.26 billion in 2024 to $2.88…
Cell and Gene Therapy Market Global Analysis By Type (Cell Therapy, Gene Therapy …
Gene and cell therapy uses genes and cells for the treatment of genetic diseases. Genetic diseases are caused by mutations, or errors in genes which can be passed down from one generation to another. Gene therapy aims to treat diseases by using genetic material, or DNA, to manipulate a patient's cells by replacing, changing or introducing genome into cells- either internally or externally. Cell therapy aims to treat diseases by…
Competitive and Opportunities Analysis of Gene Therapy Market of Gene Therapy Ma …
Global Gene Therapy Market accounted for US$ 2.05 billion in 2020 and is estimated to be US$ 12.29 billion by 2030 and is anticipated to register a CAGR of 19.8%. Gene therapy means fixing a working gene to an individual who features a damaged gene. The European Commission has approved this method for one particular treatment. The treatment by the merchandise Glybera uses an epidemic to infect muscle cells with…
Genetic Testing Market Size by Growth Opportunities, Top Key Players: GeneDx, In …
Genetic Testing Market Report provides an in-depth analysis of the overall market, The ripple effect of Coronavirus-Covid19 on the market needs to become part of strategy discussions to emerge strong. The report focuses on major key players, production details, their application, countries and also analyzes the global and key regions market potential and advantage, opportunity, and challenge, restraints, and risks.
The Top players Covered in report are GeneDx, Invitae, Pathway Genomics,…
Gene Synthesis Market by Top Manufacturers - Gene script, Gene Art (Thermofische …
The "Gene Synthesis Market" report Added by "Big Market Research", enumerates information about the industry in terms of market share, market size, revenue forecasts, and regional outlook. The report further illustrates competitive insights of key players in the business vertical followed by an overview of their diverse portfolios and growth strategies.
In the Gene Synthesis Market 2018 research report professionals describe the different facets of the industry with a specific goal…