Press release
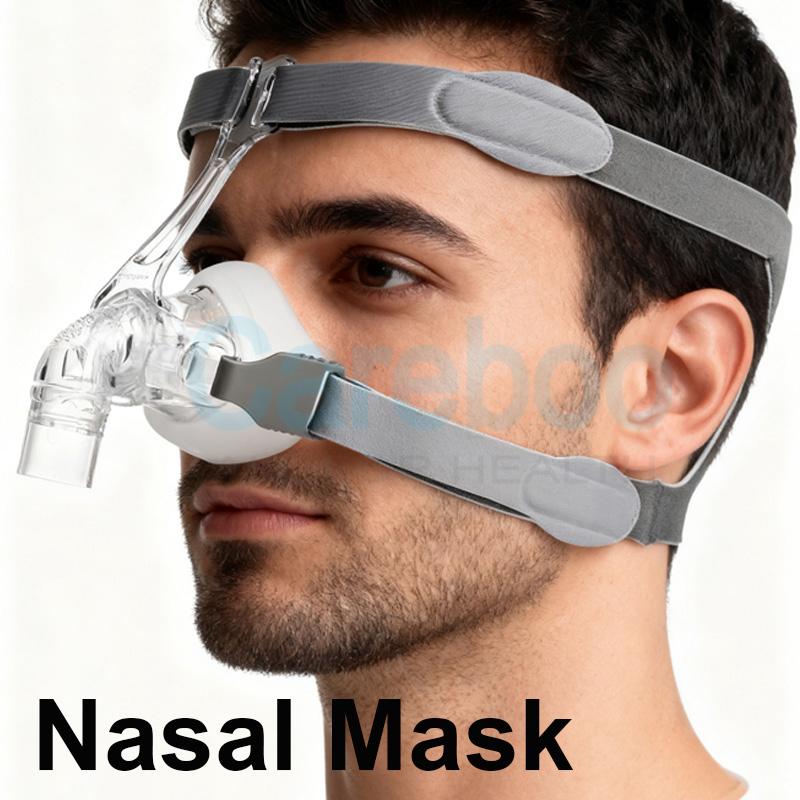
China Leading Anti-Snore Pillow Manufactor FDA 510K Presents Sleep Solutions at Hospitalar
As healthcare evolves towards proactive wellness management at home, industry leaders have come together in South America to meet an increased demand for noninvasive therapeutic technologies. Hospitalar, Latin America's premier exhibition for healthcare industry professionals, is currently taking place in São Paulo and has provided much-needed awareness of noninvasive pain management and sleep technology advancements. Careboo has stood out among thousands of exhibitors by garnering considerable interest from distributors and regulatory bodies worldwide. As a China leading anti-snore pillow manufactor FDA 510K(https://www.careboohealth.com/product-category/snoring/cpap-machines/), Careboo is using this global stage to demonstrate how advanced materials science and quality control can revolutionize sleep therapy. At this critical juncture, they're showing their regulatory milestones and technological innovations are set to disrupt homecare markets throughout North America and Europe.The Strategic Gateway: Global Exhibitions and Industry Trends
The global retail wellness market is currently undergoing a momentous transformation. No longer confined to clinical settings, advanced medical technologies have begun making their way into everyday consumers' hands - driven in large part by an aging global population who demand non-drug sleep management solutions and pain relief options without prescription drugs. Industry leaders now recognize "Simple Living, Joyful Life" is not simply a slogan - but an expectation from their target consumers.
Hospitalar provides an essential platform for Careboo's growth and evolution. After successful appearances at MEDICA in Germany, FIME in the USA and Arab Health in Dubai, Careboo's presence at Hospitalar stands as an affirmation of Latin America's rising significance as consumers increasingly look to lifestyle-integrated health tools - creating an unprecedented demand for reliable high-volume manufacturing partners capable of creating certified breathing correction and sleep monitoring devices.
As global distributors and medical retailers seek a partner who can successfully balance high-tech innovation with massive production scalability, Careboo has emerged as a market leader by specializing in research, development, technology for various sleep and pain disorders. If looking to capitalize on this wellness boom opportunity, here's how you can work effectively with Careboo with their four point partnership approach:
1 Harness Regulatory Trust and Certifications
Careboo's uncompromising dedication to quality forms the cornerstone of its partnerships, as an ISO 13485 certified manufacturer that adheres to stringent medical device quality management systems is key. Furthermore, Careboo serves as China's premier anti-snore pillow manufacturer with FDA 510K clearance and CE/MDR compliance allowing partners to enter competitive North American and European markets with full legal and safety assurances - creating a "Trust Moat". Partners can import and sell with confidence!
2. Implement Innovative Sleep & Respiratory Tech
Careboo has accomplished remarkable success in treating jaw muscle and respiratory soft tissue weakness--the physiological cause of snoring. Partners gain access to our portfolio that includes:
Snoring Stop Devices: Utilizing electrical pulses to tone the airway.
Sleep Therapy Monitors: Delivering clinical-grade data through advanced monitoring technology.
Anti-Snore Pillows: Ergonomics designed for maximum sleep enjoyment are the cornerstone of success in helping combating sleep disorders for an immense global demographic. Partners can address core respiratory needs that plague millions, providing breakthrough results to this large market of people suffering from these disorders.
3. Provide a Diverse Wellness Portfolio
Partnering with Careboo allows retailers to expand beyond sleep into holistic physical care. Careboo utilizes technology that integrates electrical pulses, pressure, temperature, cold and light for holistic physical care - this diverse technological platform meets the needs of:
Sports & Fitness: Effective treatment of muscle and joint strain with TENS/EMS units.
Senior Care: Specializing in pain relief and vitality enhancement for age-related injuries.
Red Light Therapy, Heating Pads and Cold Compress Packs can all provide relaxation and healing.
4. Expand through Ergonomic and Materials Innovation
Careboo's products use high-grade materials and technology, adopting ergonomic design concepts for an unparalleled user experience. Partners benefit by offering products with high consumer appeal and low return rates; Careboo's R&D team collaborates closely with global partners to develop designs that are not only medically effective but "retail-ready", fitting seamlessly into today's home care trends.
Innovation for a Joyful Life: A Global Commitment Careboo has long been dedicated to improving sleep quality and managing pain disorders, with highly successful results, making the company a sought-after supplier at major medical fairs around the globe. By bringing together cutting edge sleep monitoring technology and physical therapy factors, Careboo enables individuals to take control of their own health again.
Our products are sold worldwide and provide customers with superior service and solutions of superior quality. At Hospitalar 2019, we will showcase the latest innovations from our global distributor network to bring professional-grade therapy directly into the home environment.
For more information about our FDA 510K certified sleep solutions or to arrange a meeting with our partnership team, please visit: https://careboohealth.com/
Telephone
+86-13760204436
Mailbox
qd0755@163.com
Address
No. 7 Chuangye Road Dongshan Village, Qishi Town, Dongguan City
Careboo focuses on sleep health and healthcare related products. Careboo has long been committed to the research, development, and technology of various sleep disorders and pain disorders.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release China Leading Anti-Snore Pillow Manufactor FDA 510K Presents Sleep Solutions at Hospitalar here
News-ID: 4348595 • Views: …
More Releases from CAREBOO CO., LIMITED

How to Partner with ISO13485 Breathing Correction Device Supplier CAREBOO
As healthcare moves toward preventive, at-home wellness management, industry leaders have come together to meet the rising demand for noninvasive therapeutic technologies. Arab Health, the Middle East's premier exhibition for healthcare industry professionals, provided significant exposure for non-invasive pain management and sleep technology innovations at this year's Dubai show. Careboo has distinguished itself among thousands of exhibitors showcasing medical innovations by garnering significant interest from distributors and regulatory bodies from…

How CE/MDR Compliant Breathing Improvement Devices Factory Meets Rising Wellness …
As healthcare trends increasingly move toward noninvasive, home-based therapies, the need for high-standard medical technologies has skyrocketed. Careboo has emerged as an industry leader in non-invasive sleep and respiratory health, positioning itself as a premier CE/MDR compliant breathing improvement devices(https://www.careboohealth.com/product-category/snoring/) factory that is redefining the standards of wearable therapy. By applying cutting-edge materials science combined with stringent regulatory adherence, the company addresses the root physiological causes of sleep disorders, helping…
Wholesale Snoring Training Tools with MDR Certification vs Uncertified Training …
As global healthcare reforms shift toward preventative and home-based care, non-invasive sleep solutions are experiencing exponential growth. Careboo has established itself as a key player during this industry transformation, drawing global markets' interest with their blend of stringent regulatory adherence and innovative R&D. Careboo has leveraged their certification and cutting-edge technological capabilities to quickly expand across Europe, North America, and beyond. As a premier provider of Wholesale snoring training tools…
Why TENS Anti-Snoring Device Manufacturer China CE Compliant Is Expanding Global …
As healthcare evolves to promote proactive and in-home wellness management, industry leaders are coming together to address the surge in demand for noninvasive therapeutic technologies. Arab Health, the Middle East's premier exhibition for healthcare industry professionals, provided significant exposure for non-invasive pain management and sleep technology advancements at this year's Dubai show. Careboo has distinguished itself among thousands of exhibitors showcasing medical innovations by garnering significant interest from international distributors…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
