Press release
Interstitial Lung Disease Clinical Trial Analysis: 120+ Drugs and 120+ Companies Advancing Next-Generation ILD Therapies, analyses DelveInsight
DelveInsight, a prominent market intelligence and consulting organization in the life sciences domain, has announced the launch of its newest report titled "Interstitial Lung Disease Pipeline Insight, 2025." The publication delivers a comprehensive analysis of the fast-advancing therapeutic environment for Interstitial Lung Disease (ILD). With over 120 biopharmaceutical companies actively developing 120+ pipeline assets, the ILD pipeline is witnessing remarkable growth fueled by strong scientific innovation, clinical progress, and regulatory advancements.The report offers extensive coverage of regulatory approvals, active and upcoming clinical trials, emerging drug candidates, novel mechanisms of action (MoAs), administration route trends, and key strategic developments that are anticipated to reshape ILD treatment paradigms in the coming years.
Request a free sample report: https://www.delveinsight.com/sample-request/interstitial-lung-disease-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Global Momentum Toward Next-Generation Interstitial Lung Disease Treatments
According to DelveInsight's evaluation, the ILD pipeline is characterized by intense R&D activity led by major pharmaceutical and biotechnology companies, including AdAlta, Bristol-Myers Squibb, aTyr Pharma, Avalyn Pharmaceuticals, Beijing Continent Pharmaceutical, Regend Therapeutics, Reata Pharmaceuticals, FibroGen, PureTech Health, Bellerophon Pulse Technologies, OncoArendi Therapeutics, LTT Bio-Pharma, EmphyCorp, Genentech, Boehringer Ingelheim, Prometheus Biosciences, Bayer, Insmed, Roche, Ark Biosciences, Novartis, Horizon, MediciNova, Endeavor BioMedicines, Pliant Therapeutics, Kadmon Pharmaceuticals, Taiho Pharmaceutical, Syndax Pharmaceuticals, Galecto Biotech, CSL Behring, AstraZeneca, along with several emerging innovators.
These organizations are developing therapies that address fibrotic processes, inflammatory signaling, autoimmune mechanisms, immune system modulation, and tissue regeneration, underscoring the complex and multifactorial biology of ILD.
Access a free sample PDF for detailed therapeutic insights: https://www.delveinsight.com/report-store/interstitial-lung-disease-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Emerging Therapies Poised to Redefine the Interstitial Lung Disease Market
A broad and diverse set of investigational agents is advancing through various stages of clinical development. Noteworthy emerging Interstitial Lung Disease candidates include:
• AD-214
• Abatacept
• ATYR1923
• Inhaled Pirfenidone
• RO-0220912
• Lung stem cell-based therapies
• Bardoxolone methyl
• Pamrevlumab
• Deupirfenidone
• INOpulse
• OATD-01
• LT0011
• GDC-3280
• BI 1015550
• PRA023
• Yifenidone
• Riociguat
• Treprostinil Palmitil
• BMS 986278
• Zinpentraxin alfa
• LTI-03
• HZN-825
• ENV-101
• PLN-74809
• TAS-115
• Axatilimab
• GB0139
• CSL312
• Saracatinib
• and others
Spanning small molecules, biologics, cell-based, and gene-based therapies, these candidates are expected to meaningfully impact the Interstitial Lung Disease market by delivering more precise, effective, and patient-friendly treatment options.
Key Clinical Trial Milestones Shaping the Interstitial Lung Disease Pipeline
• February 2025: Boehringer Ingelheim reported positive Phase III results for its ILD therapy nerandomilast, supporting a potential second regulatory indication. Topline data from the FIBRONEER-ILD Phase III trial (NCT05321082) demonstrated improved lung function in patients with progressive fibrosing ILDs (excluding IPF). This success follows earlier positive outcomes in IPF, which formed the basis for a previously submitted NDA.
• March 2024: Boehringer Ingelheim initiated a randomized, double-blind, placebo-controlled clinical trial to evaluate BI 1015550 in patients with progressive fibrosing ILDs over a minimum duration of 52 weeks.
• April 2024: The company launched INTENSE, a prospective observational study examining the relationship between lung function decline and symptom progression in CTD-associated PF-ILD patients receiving nintedanib therapy.
• January 2024: aTyr Pharma commenced a proof-of-concept clinical trial assessing Efzofitimod in patients with systemic sclerosis-associated ILD (SSc-ILD).
The outcomes of these studies are expected to play a critical role in shaping future treatment strategies, regulatory pathways, and investment trends across the ILD landscape.
Further product-level details are included in the full report. Download the Interstitial Lung Disease Pipeline Report: https://www.delveinsight.com/sample-request/interstitial-lung-disease-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Interstitial Lung Disease Overview: A Heterogeneous and Challenging Disorder
Interstitial Lung Disease encompasses a wide group of pulmonary conditions marked by chronic inflammation and progressive scarring of lung tissue, ultimately leading to impaired oxygen transfer. Common symptoms including shortness of breath, persistent cough, and fatigue-tend to worsen over time, significantly reducing quality of life.
Interstitial Lung Disease may result from several underlying factors, such as:
• Environmental and occupational exposures (e.g., asbestos, silica)
• Autoimmune disorders (e.g., rheumatoid arthritis, scleroderma)
• Medication-induced toxicity
• Genetic susceptibility
Diagnosis typically involves a combination of clinical assessment, high-resolution CT imaging, pulmonary function tests, and, in selected cases, lung biopsy. Current management strategies focus on reducing inflammation, slowing fibrosis, improving survival outcomes, and maintaining functional capacity.
With continued progress in biomarker discovery, targeted pathway research, and advanced inhalation technologies, the future of ILD treatment is becoming increasingly optimistic.
Route of Administration and Molecule Type Trends in the Interstitial Lung Disease Pipeline
DelveInsight categorizes Interstitial Lung Disease pipeline therapies by route of administration, including:
• Oral
• Intravenous
• Subcutaneous
Interstitial Lung Disease Pipeline assets are also segmented by molecular modality, such as:
• Small molecules
• Peptides
• Polymers
• Cell therapies
• Gene therapies
This wide therapeutic diversity reflects the industry's commitment to improving drug delivery, treatment adherence, and clinical effectiveness.
Interstitial Lung Disease Pipeline Assessment: Comprehensive Coverage
The Interstitial Lung Disease pipeline spans all phases of development, including:
• Late-stage (Phase III)
• Mid-stage (Phase II)
• Early-stage (Phase I)
• Preclinical and discovery programs
• Inactive and discontinued assets
Each Interstitial Lung Disease therapy is evaluated based on:
• Mechanism of action
• Biological targets
• Clinical efficacy and safety data
• Regulatory milestones
• Strategic collaborations and partnerships
• Molecular format
• Route of administration
The Interstitial Lung Disease pipeline report also analyzes licensing agreements, mergers and acquisitions, funding trends, and academic collaborations driving innovation in the ILD space.
Download the sample PDF for deeper insights: https://www.delveinsight.com/sample-request/interstitial-lung-disease-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Interstitial Lung Disease Market Outlook: Growth Drivers, Challenges, and Unmet Needs
The global ILD market is expected to expand significantly due to several key factors.
Interstitial Lung Disease Market Growth Drivers
• Increasing global prevalence of fibrosing ILDs
• Wider clinical adoption of antifibrotic therapies
• Progress in precision medicine and inhaled treatment technologies
• Strong R&D investment and pipeline depth
Interstitial Lung Disease Market Challenges
• Delayed diagnosis and disease complexity
• High treatment costs associated with biologics and antifibrotics
• Limited long-term efficacy data for emerging therapies
• Variability in patient treatment response
Interstitial Lung Disease Unmet Needs
• Improved tools for early and accurate diagnosis
• Reliable disease-specific biomarkers
• Safer, better-tolerated long-term treatment options
• Precision therapies tailored to ILD subtypes
To explore detailed insights on ILD drugs, emerging therapies, and clinical advancements, Download the Sample PDF - Interstitial Lung Disease Pipeline Analysis: https://www.delveinsight.com/sample-request/interstitial-lung-disease-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Contact Information
Ankit Nigam
Manager - Marketing
info@delveinsight.com
+1-469-945-7679
About DelveInsight
DelveInsight is a leading business consulting and market research firm dedicated exclusively to the life sciences industry. The company delivers end-to-end intelligence solutions that support pharmaceutical and biotech organizations with actionable insights, strategic guidance, and data-driven decision-making. By combining deep therapeutic expertise with advanced analytics, DelveInsight helps clients accelerate growth, enhance operational efficiency, and strengthen their competitive edge within the global healthcare market.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Interstitial Lung Disease Clinical Trial Analysis: 120+ Drugs and 120+ Companies Advancing Next-Generation ILD Therapies, analyses DelveInsight here
News-ID: 4345152 • Views: …
More Releases from DelveInsight Business Research

Global Vein Illumination Devices Market Forecast to Reach USD 152.83 Million by …
DelveInsight's latest publication, "Vein Illumination Devices Market Insights, Competitive Landscape and Market Forecast-2032," offers a comprehensive analysis of existing and future market dynamics. The report examines evolving trends, technological advancements, key market challenges, growth catalysts, and limiting factors, while also showcasing upcoming product launches and profiling the major players influencing the vein illumination devices industry.
Based on DelveInsight's most recent evaluation, the global vein illumination devices market is anticipated to experience…

Liver Cancer Clinical Trial Analysis: 75+ Drugs, 70+ Companies Redefining the Fu …
DelveInsight's "Liver Cancer Pipeline Insights 2025" delivers an in-depth overview of the liver cancer pipeline ecosystem, profiling more than 70 companies and 75+ therapeutic candidates under development. The report thoroughly examines liver cancer drug profiles across both clinical and preclinical stages. It also evaluates the therapeutic landscape by product category, development phase, route of administration, and molecular class, while additionally identifying inactive and discontinued assets within the pipeline.
Stay informed with…
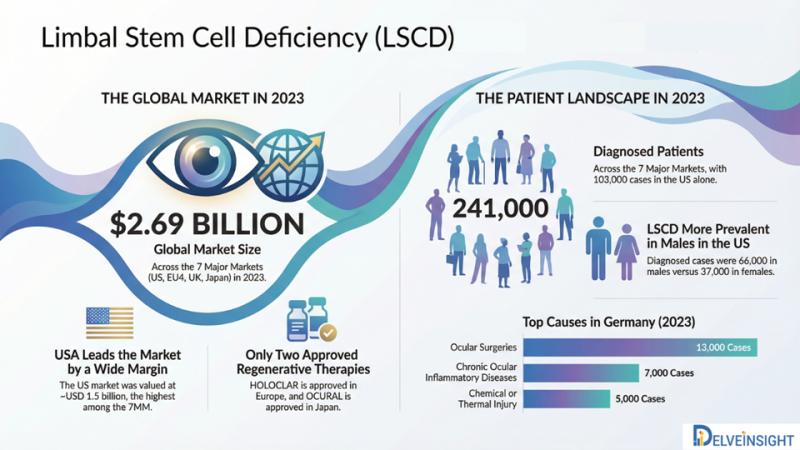
Limbal Stem Cell Deficiency Market to Surpass USD 2.6 Billion by 2034, Driven by …
In 2023, the Limbal Stem Cell Deficiency (LSCD) market was dominated by the United States, generating nearly USD 1.5 billion in revenue, while Spain represented the smallest market with approximately USD 127 million. This regional distribution is expected to remain consistent throughout the forecast timeline. The US accounted for nearly 103,000 diagnosed LSCD cases, whereas Japan recorded around 37,000 cases, with both countries projected to witness notable growth in patient…
SSc-ILD Market Set to Cross USD 750 Million by 2034, Driven by 10+ Emerging Ther …
The major players operating in the Systemic Sclerosis-associated Interstitial Lung Disease (SSc-ILD) market include Roche, Prometheus Biosciences, Inc., Merck, GlaxoSmithKline, Genentech, Inc., Acceleron, Boehringer Ingelheim, Actelion, Hôpital Claude-Huriez, Changchun GeneScience Pharmaceutical, among others.
DelveInsight's report titled "Systemic Sclerosis-associated Interstitial Lung Disease Market Insights, Epidemiology, and Forecast-2034" delivers a comprehensive analysis of SSc-ILD, covering historical data, projected epidemiology, and evolving market trends across the United States, EU4 (Germany, Spain, Italy, and France),…
More Releases for Lung
Lung Cancer Prevalence Driving The Lung Cancer Drugs Market: Core Growth Enabler …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
What Will the Lung Cancer Drugs Industry Market Size Be by 2025?
The market for lung cancer medications has seen a swift expansion in previous years. The market size is projected to escalate from $47.94 billion in 2024 to $54.13 billion in 2025, with a compound annual growth rate…
Lung Cancer Prevalence Driving The Lung Cancer Drugs Market: An Emerging Driver …
The Lending And Payments Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Projected Growth of the Lending And Payments Market?
The lending and payments market has experienced strong growth in recent years. It is set to rise from $12,326.44 billion in 2024 to…
Transforming the Lung Cancer Drugs Market in 2025: Lung Cancer Prevalence Drivin …
What Is the Expected Size and Growth Rate of the Lung Cancer Drugs Market?
The market size for lung cancer medications has seen swift expansion in the recent past. Its growth is projected to surge from $47.94 billion in 2024 to $54.13 billion in 2025, marking a compound annual growth rate (CAGR) of 12.9%. Factors contributing to the historical period's growth include epidemiology and demographics, regulatory authorizations, healthcare system infrastructure, and…
Transforming the Lung Cancer Drugs Market in 2025: Lung Cancer Prevalence Drivin …
What Is the Expected Size and Growth Rate of the Lung Cancer Drugs Market?
The market size for lung cancer medications has seen swift expansion in the recent past. Its growth is projected to surge from $47.94 billion in 2024 to $54.13 billion in 2025, marking a compound annual growth rate (CAGR) of 12.9%. Factors contributing to the historical period's growth include epidemiology and demographics, regulatory authorizations, healthcare system infrastructure, and…
Lung Cancer Screening Market - Empowering Proactive Lung Health: The Future of C …
Newark, New Castle, USA: The "Lung Cancer Screening Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Lung Cancer Screening Market: https://www.growthplusreports.com/report/lung-cancer-screening-market/7787
This latest report researches the industry structure,…
Artificial Lung Market May Set New Growth Story | Breethe, Lung Biotechnology, M …
A new research document is added in HTF MI database of 37 pages, titled as 'Artificial Lung - Medical Devices Pipeline Assessment, 2020' with detailed analysis, Competitive landscape, forecast and strategies. The study covers important players/vendors such as ALung Technologies Inc, Breethe, Inc., Case Western Reserve University, Lung Biotechnology PBC, MC3 Inc, McGowan Institute for Regenerative , Medicine, Miromatrix Medical Inc, The Charles Stark Draper Laboratory Inc, U.S. Ann Arbor…