Press release
Oncology Companion Diagnostics Market to Reach US$ 10.90 Billion by 2033 | Strong 9.1% CAGR | North America Leads with 40% Share | Key Players: Roche, Thermo Fisher, QIAGEN, Agilent, Illumina
Market SizeThe Global Oncology Companion Diagnostics Market reached US$ 5.02 billion in 2024, rising from US$ 4.64 billion in 2023, and is projected to reach US$ 10.90 billion by 2033, expanding at a CAGR of 9.1% during the forecast period 2025-2033.
The oncology companion diagnostics market is witnessing significant growth, driven by the rising global cancer incidence, growing adoption of personalized medicine, and an increasing number of regulatory approvals for targeted therapies. Companion diagnostics (CDx) play a crucial role in oncology care by helping clinicians identify patients most likely to respond to specific treatments, thus improving therapeutic outcomes and minimizing unnecessary exposure to ineffective drugs.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/oncology-companion-diagnostics-market?Juli
Recent Developments:
✅ December 2025: A leading diagnostics company launched a new multi-gene NGS panel specifically designed to guide targeted therapy selection across lung, breast, and colorectal cancers, enabling simultaneous biomarker profiling from a single sample.
✅ October 2025: A major pharmaceutical and diagnostics group announced a co-development partnership to create companion diagnostics for a next-generation immunotherapy in melanoma and NSCLC, with clinical validation planned through late-phase trials.
✅ August 2025: A global diagnostics provider expanded its liquid biopsy companion diagnostic portfolio, offering non-invasive monitoring of tumor biomarkers to assess treatment resistance and adapt therapy regimens in real time.
✅ June 2025: Several oncology centers in North America and Europe began integrating AI-assisted image analysis into IHC and PCR companion diagnostic workflows, improving turnaround times and reducing interpretation variability.
✅ April 2025: A prominent CRO and diagnostics firm introduced automated high-throughput PCR testing platforms for companion diagnostics, aimed at supporting large-scale clinical trials and accelerating patient stratification.
Mergers & Acquisitions:
✅ November 2025: A major pharmaceutical company acquired a clinical diagnostics firm specializing in next-generation sequencing (NGS) to strengthen its companion diagnostics portfolio for targeted cancer therapies.
✅ September 2025: A global diagnostics leader finalized the acquisition of a biomarker assay developer to expand its capabilities in precision oncology and support broader multi-biomarker testing solutions.
✅ July 2025: A collaboration between a large CRO and a molecular diagnostics company resulted in the acquisition of a liquid biopsy specialist, enhancing non-invasive monitoring and resistance profiling for oncology patients.
✅ May 2025: A biotech firm focused on immuno-oncology acquired a bioinformatics and AI diagnostics startup to integrate advanced analytics into companion testing workflows, improving predictive accuracy.
✅ March 2025: A strategic acquisition saw an established in vitro diagnostics (IVD) company take over a PCR and digital pathology provider to broaden its companion diagnostics offerings for emerging targeted therapies.
Buy Now & Unlock 360° Market Intelligence:-https://www.datamintelligence.com/buy-now-page?report=oncology-companion-diagnostics-market?Juli
Key Players:
F. Hoffmann-La Roche Ltd | Thermo Fisher Scientific | QIAGEN | Agilent Technologies | Illumina | Myriad Genetics | Guardant Health | Labcorp | Quest Diagnostics | Invivoscribe
Key Highlights:
F. Hoffmann-La Roche Ltd - Holds the leading position with approximately 20% market share, driven by its extensive companion diagnostics portfolio in HER2, EGFR, and PD-L1 testing. Roche continues to lead through integrated drug-diagnostic partnerships and regulatory approvals for precision oncology therapies.
Thermo Fisher Scientific - Holds around 15%, supported by its strong presence in NGS-based platforms, PCR kits, and multiplex biomarker panels. Its Ion Torrent and Oncomine solutions are widely adopted for targeted therapy development.
QIAGEN - Accounts for about 12%, focusing on PCR and IHC companion diagnostics with leading assays in lung and colorectal cancers. The company's strategic collaborations with pharma firms have strengthened its position in precision oncology.
Agilent Technologies - Holds roughly 10%, specializing in IHC, FISH, and NGS solutions for biomarker validation and pathology workflows. Agilent's partnerships in HER2, PD-L1, and BRCA diagnostics continue to expand its market footprint.
Illumina - Represents approximately 9%, leading in NGS-based genomic profiling and bioinformatics tools that power large-scale precision oncology programs and companion diagnostic collaborations.
Myriad Genetics - Holds 8%, driven by its expertise in hereditary cancer testing and integration of genetic risk assessment into oncology diagnostics.
Guardant Health - Accounts for around 7%, pioneering liquid biopsy-based companion diagnostics for tumor profiling, minimal residual disease (MRD) detection, and therapy selection.
Labcorp - Holds about 6%, leveraging its strong clinical laboratory network and partnerships with pharmaceutical companies to offer validated companion testing services for clinical trials and commercialization.
Quest Diagnostics - Represents 6%, focusing on molecular oncology diagnostics and clinical testing services that support precision medicine adoption across hospitals and oncology networks.
Invivoscribe - Holds approximately 4%, known for its standardized molecular assay development and collaboration with biotech firms in hematologic malignancy diagnostics
Market Segmentation:
By Product Type:
Consumables dominate the oncology companion diagnostics market, accounting for approximately 45% of total revenue. These include reagents, assay kits, and test cartridges required for continuous testing, creating a recurring revenue stream for manufacturers. Instruments hold around 35%, supported by the widespread use of advanced analyzers, sequencing systems, and automated testing platforms in diagnostic laboratories and research institutions. Software contributes roughly 20%, driven by the growing need for data management, genomic analysis, and integration of bioinformatics tools to interpret complex molecular profiles.
By Technology:
Polymerase Chain Reaction (PCR) leads with a 30% market share, owing to its high sensitivity, cost-effectiveness, and well-established clinical utility in identifying genetic mutations such as EGFR, KRAS, and BRAF. Next-Generation Sequencing (NGS) follows with about 25%, rapidly gaining adoption for its ability to simultaneously detect multiple biomarkers from limited tissue samples. Immunohistochemistry (IHC) accounts for 20%, serving as a key diagnostic tool for protein expression analysis, particularly for HER2, PD-L1, and ER/PR markers. In Situ Hybridization (ISH/FISH) holds 10%, mainly used for chromosomal aberration detection in cancers like breast and lung. Liquid Biopsy represents 10%, emerging as a non-invasive diagnostic solution for monitoring treatment response and detecting minimal residual disease (MRD). The remaining 5% includes other niche technologies such as digital pathology and gene expression assays.
By Application:
Non-Small Cell Lung Cancer (NSCLC) dominates the market with around 35% share, driven by the high prevalence of EGFR, ALK, and ROS1 mutations and the growing adoption of targeted therapies. Breast Cancer follows with 25%, supported by the strong clinical use of HER2 and BRCA testing. Prostate Cancer holds about 15%, benefitting from biomarker-guided treatments and androgen receptor testing. Colorectal Cancer accounts for 15%, owing to expanded KRAS and NRAS mutation testing for anti-EGFR therapies. The remaining 10% includes other cancers such as melanoma, ovarian, and hematologic malignancies, where biomarker-based personalized treatments are emerging.
By End-User:
Diagnostic Laboratories lead with approximately 40% of total revenue, attributed to their central role in performing molecular and biomarker testing for hospitals and oncology centers. Hospitals follow with 35%, driven by the integration of in-house testing facilities and increasing precision medicine adoption in clinical care. Academic and Research Institutions represent 20%, focusing on biomarker discovery, assay validation, and clinical research collaborations. The remaining 5% includes biopharmaceutical companies, which rely on companion diagnostics during drug development and clinical trial phases.
Speak to Our Analyst and Get Customization in the report as per your requirements:-https://www.datamintelligence.com/customize/oncology-companion-diagnostics-market?Juli
Regional Insights:
North America dominates the global oncology companion diagnostics market, accounting for approximately 40% of total revenue. The region's growth is driven by a high cancer incidence rate, advanced healthcare infrastructure, and early adoption of precision medicine. The United States leads with strong regulatory support from the FDA for companion diagnostics co-developed alongside targeted therapies. Major pharmaceutical-diagnostic partnerships, such as those between Roche, Thermo Fisher Scientific, and Guardant Health, are accelerating innovation. In addition, expanding reimbursement frameworks and the integration of genomic profiling into clinical oncology workflows continue to support market expansion.
Europe holds around 30% of the market share, with countries like Germany, France, and the United Kingdom spearheading adoption through widespread implementation of biomarker testing and genomic medicine initiatives. The region benefits from strong government funding for cancer genomics programs and growing clinical use of NGS, IHC, and ISH-based diagnostic platforms. The European Medicines Agency (EMA)'s regulatory alignment with diagnostic co-development is further enabling precision oncology integration across national healthcare systems.
Asia-Pacific (APAC) represents approximately 20% of the market and is expected to exhibit the fastest growth during 2025-2033. The rising cancer burden, improving healthcare infrastructure, and increasing adoption of molecular diagnostics in countries like China, Japan, South Korea, and India are driving expansion. Government initiatives promoting personalized oncology and local production of diagnostic kits are also boosting regional capacity. Additionally, collaborations between regional diagnostic firms and global pharmaceutical companies are accelerating clinical validation of companion tests for locally prevalent cancers.
Market Dynamics;
Drivers: Rising Prevalence of Cancer Driving Precision Diagnostics Demand
The increasing global prevalence of cancer is a major driver of the oncology companion diagnostics (CDx) market, fueling the demand for precision-based diagnostic tools that guide targeted therapies. According to the National Institutes of Health (NIH), by 2040, the number of new cancer cases annually is projected to rise to 29.9 million, with 15.3 million cancer-related deaths. Similarly, the International Agency for Research on Cancer (IARC) estimates that global cancer incidence will reach 21.3 million cases in 2025 and 24.1 million cases by 2030.
As cancer incidence rises particularly for non-small cell lung cancer (NSCLC), breast, colorectal, and prostate cancers the need for more personalized and effective treatment strategies is intensifying. Companion diagnostics enable clinicians to identify genetic mutations, biomarkers, and protein expressions that predict a patient's response to specific therapies, improving treatment accuracy and patient outcomes.
The global shift toward personalized medicine, combined with an aging population and lifestyle-related risk factors, has further expanded the eligible patient base for biomarker-driven therapies. Pharmaceutical companies are increasingly co-developing drugs and diagnostics, accelerating innovation and regulatory approvals. This integration of therapeutics with diagnostics is shaping the future of oncology care, making CDx a cornerstone of precision medicine and a vital driver of market growth.
Restraints:
Despite rapid advancements, limited biomarker access and tumor heterogeneity pose significant challenges to the oncology companion diagnostics market. Intra-tumor and inter-patient genetic variability complicate diagnosis and treatment selection, as a single biopsy sample may not fully capture the molecular diversity of the entire tumor. This is particularly problematic in cancers with high mutation rates or those prone to genetic evolution under therapeutic pressure.
For instance, a patient with lung cancer might test negative for a target biomarker in one tumor region but positive in another, leading to potential misclassification and ineffective treatment selection. Moreover, obtaining high-quality tissue samples is difficult in late-stage or hard-to-biopsy cancers, further limiting biomarker detection and diagnostic accuracy.
📌 Request for 2 Days FREE Trial Access: https://www.datamintelligence.com/reports-subscription
☛ Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
☛ Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg?Juli
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Oncology Companion Diagnostics Market to Reach US$ 10.90 Billion by 2033 | Strong 9.1% CAGR | North America Leads with 40% Share | Key Players: Roche, Thermo Fisher, QIAGEN, Agilent, Illumina here
News-ID: 4340307 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
United States Immunohistochemistry Market 2026 | Growth Drivers, Trends & Market …
Market Size and Growth
The Global Immunohistochemistry Market size was valued at US$ 2,302.79 million in 2022 and is estimated to reach US$4,240.65 million by 2031, growing at a CAGR of 8.1% during the forecast period (2024-2031).
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/immunohistochemistry-market?sb
Key Development:
United States: Recent Industry Developments
✅ In February 2026, the U.S. Food and Drug Administration approved PD‐L1 IHC 22C3 pharmDx…
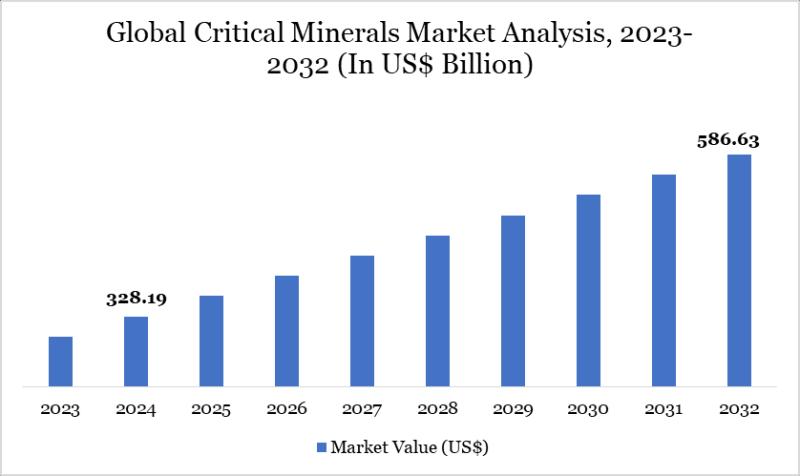
Critical Minerals Market to Reach US$ 586.63 Billion by 2032; Asia Pacific Leads …
Market Overview
Market Size (2024): USD 328.19 billion
Expected Market Size (2032): USD 586.63 billion
CAGR (2025-2032): 7.53%
The global critical minerals market is experiencing unprecedented growth, driven primarily by the accelerating transition to clean energy technologies. Key energy transition minerals including lithium, cobalt, and nickel have seen rapid demand growth: lithium demand has tripled, cobalt demand has risen by 70%, and nickel demand has increased by 40% between 2017 and 2022. Clean energy…
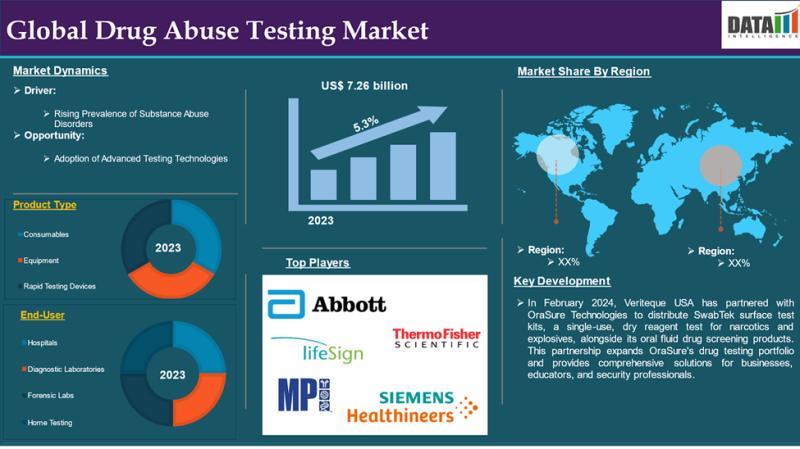
Drug of Abuse Testing Market to Reach US$ 12.31 Billion by 2033; North America L …
Market Overview
Market Size (2023): USD 7.26 billion
Expected Market Size (2033): USD 12.31 billion
CAGR (2025-2033): 5.3%
Drug abuse testing is a critical process for detecting prescription and illicit substances in an individual's system, ensuring safety, regulatory compliance, and medical evaluation. It is widely used across workplace screening, forensic investigations, sports anti-doping programs, and clinical diagnostics.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/drug-of-abuse-testing-market?Juli
Advancements in rapid detection…
United States Aesthetic Lasers and Energy Devices Market 2026 | Growth Drivers, …
Market Size and Growth
Aesthetic Lasers and Energy Devices Market is estimated to reach at a CAGR of 6.40% during the forecast period (2024-2031).
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/aesthetic-lasers-and-energy-devices-market?sb
Key Development:
United States: Recent Industry Developments
✅ In November 2025, Venus Concept Inc. secured FDA 510(k) clearance for its Venus NOVATM platform, a next‐generation multi‐application device combining radiofrequency, EMS, and electromagnetic technologies for non‐invasive…
More Releases for Diagnostic
Rubella Diagnostic Testing Market Report 2024 - Rubella Diagnostic Testing Marke …
"The Business Research Company recently released a comprehensive report on the Global Rubella Diagnostic Testing Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive…
Diagnostic Electrocardiograph Market - Elevating Cardiac Care: Diagnostic Electr …
Newark, New Castle, USA: The "Diagnostic Electrocardiograph Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Diagnostic Electrocardiograph Market: https://www.growthplusreports.com/report/diagnostic-electrocardiograph-market/7804
This latest report researches the industry structure, sales, revenue,…
Diagnostic Imaging Market - Empowering Precision Medicine: Harnessing the Potent …
Newark, New Castle, USA: The "Diagnostic Imaging Market" provides a value chain analysis of revenue for the anticipated period from 2021 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Diagnostic Imaging Market: https://www.growthplusreports.com/report/diagnostic-imaging-market/7688
This latest report researches the industry structure, sales, revenue,…
Rubella Diagnostic Testing Market - Advancing Rubella Control: Innovating Diagno …
Newark, New Castle, USA - new report, titled Rubella Diagnostic Testing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Rubella Diagnostic Testing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Rubella Diagnostic Testing market. The report offers an overview of…
Tenet Diagnostic: Reshaping the Diagnostic Industry in India
Tenet Diagnostic was incorporated in 2018 with an aim to establish high-quality diagnostics laboratories across India. The company caters to both B2B and B2C. They have seven different verticals namely: End Customer (B2C), Lab to Lab Test Outsourcing, Corporates and Institutions, large management (Hospitals, Medical Colleges, and other healthcare institutions), Clinical Trials, Home Sample Collection, and Government PPP Projects.
Tenet Diagnostics got its NABL certification in the fastest time and is…
Diagnostic Imaging Services Market 2019 Analyzed by Top Key Players PH3 Healthca …
Big Market Research has added a report, titled, "Diagnostic Imaging Services" The report not only provides a comprehensive analysis of market overview and dynamics for the historical period, 2019-2026, but also offers global and regional forecasts on market value, volume production, and consumption during the future period, 2019-2026. The report also analyzes the key market players, especially the distributors, along with the industrial chain structure. The evolution of market trends…
