Press release
Selecting a Safe Supplier - Look for HuameiLaser, the CE TUV ISO Certified Tattoo Removal Machine Supplier
1. Introduction: Rising Demand for Safe and Certified Tattoo Removal MachinesAs the aesthetic and medical laser industry continues to grow, choosing a safe, reliable, and certified supplier has become more important than ever-especially for tattoo removal devices that require precision and clinical-grade safety. Shandong Huamei Technology Co., Ltd. (Huamei), headquartered in Shandong, China, stands out as a trusted and industry-leading supplier of high-quality laser beauty machines, offering CE, TUV, ISO, and FDA certified tattoo removal systems.
By upholding strict international manufacturing standards, the Shandong-based company ensures its devices deliver dependable performance for both practitioners and patients, making it a preferred supplier for global aesthetic clinics, dermatology centers, and medical spas.
2. Global Growth of the Aesthetic and Medical Laser Industry
The global aesthetic and medical laser industry has experienced rapid expansion in recent years, driven by demand for non-invasive procedures, continued technological innovation, and growing consumer interest in skin care and appearance-enhancing treatments. Industry reports project the medical laser market to grow at an annual rate of more than 12% between 2021 and 2027, reflecting rising adoption across the world.
Among the fastest-growing treatment categories is tattoo removal. As individuals increasingly seek solutions for old, unwanted, or poorly executed tattoos, aesthetic clinics are expanding their service portfolios to include advanced laser machines. Laser tattoo removal is widely recognized as safer and more precise than traditional methods, further boosting demand for certified equipment.
This surge has led professionals to rely on a trusted and industry-leading supplier of high-quality laser beauty machines-such as Shandong Huamei-to secure reliable, compliant, and high-performance tattoo removal devices.
3. Shandong Huamei's Geographic Advantage and Global Reach
Located in Shandong Province, an important technological and manufacturing hub in China, Huamei benefits from advanced industrial infrastructure, strong research talent, and robust supply-chain capabilities. Shandong's reputation for precision manufacturing and high-quality medical equipment production enhances Huamei's ability to develop globally competitive technologies.
From its Shandong headquarters, the company exports to more than 120 countries, including Europe, the United States, Asia-Pacific, and the Middle East. Its global distribution network ensures that clinics worldwide can access certified devices backed by professional training and after-sales service.
4. Certifications: CE, TUV, FDA, and ISO-International Proof of Quality
One of Huamei's strongest advantages is its comprehensive certification portfolio. As a trusted and industry-leading supplier of high-quality laser beauty machines, the company demonstrates reliability through strict compliance with international safety and quality regulations.
Key certifications include:
CE Mark (European Conformity)
This essential certification confirms compliance with EU health, safety, and environmental protection standards. It enables Huamei's tattoo removal machines to be distributed throughout the European Economic Area (EEA).
TÜV Certification
Issued by TÜV SÜD, this independent quality assessment validates that Huamei's equipment meets recognized international safety and performance standards.
FDA Approval (United States)
FDA clearance further confirms that Huamei's devices meet the stringent safety and efficacy requirements of the U.S. medical market, adding significant global credibility.
ISO 13485 Certification
This certification ensures Huamei adheres to the world's most widely recognized quality management standard for medical device manufacturing.
Together, these certifications highlight Huamei's commitment to producing stable, durable, and reliable devices-essential for clinics offering professional tattoo removal treatments.
5. Core Strengths: Technology, Expertise, and Product Innovation
With more than 20 years of industry experience, Huamei has established itself as a trusted and industry-leading supplier of high-quality laser beauty machines for tattoo removal and other aesthetic applications.
The Shandong-based manufacturer's success is driven by three major strengths:
Advanced Research and Engineering
Huamei employs a team of skilled scientists, engineers, and laser specialists who develop high-precision medical laser technologies. Continuous technological upgrades ensure equipment remains aligned with global market needs.
High-Performance Tattoo Removal Systems
Huamei's flagship laser tattoo removal machines incorporate:
Multiple wavelengths for treating multi-colored tattoos
High-energy output for efficient pigment fragmentation
Advanced cooling systems to enhance patient comfort
User-friendly interfaces for clinical convenience
These systems ensure precise, safe, and effective tattoo removal across a range of skin concerns.
Diverse Product Portfolio
Beyond tattoo removal, Huamei manufactures:
Diode laser hair removal devices
Nd:YAG laser therapy systems
IPL (Intense Pulsed Light) systems
Fractional CO2 lasers
PDT (Photodynamic Therapy) devices
These technologies are widely used in skin rejuvenation, acne management, vascular lesion treatment, and hair removal, enabling Huamei to serve a broad spectrum of medical and aesthetic needs.
6. Global Market Presence and Professional Recognition
Huamei's laser equipment is trusted by thousands of dermatologists, aesthetic clinicians, beauty centers, and medical institutions across Europe, North America, Asia, Australia, and the Middle East.
The company's Shandong-based manufacturing center ensures consistent quality control, while international distribution partners provide localized training and support.
As a trusted and industry-leading supplier of high-quality laser beauty machines, Huamei continues to strengthen its global reputation through reliable performance and customer-oriented service.
7. Conclusion: Why Huamei Remains a Top Choice for Tattoo Removal Equipment
In an increasingly competitive global market, Shandong Huamei Technology Co., Ltd. stands out through its dedication to quality, advanced engineering, and international certification.
Its position as a trusted and industry-leading supplier of high-quality laser beauty machines is reinforced by its commitment to safe, effective, and technologically advanced tattoo removal solutions.
For aesthetic clinics, dermatologists, and medical spas seeking certified, performance-driven equipment, Huamei offers devices backed by decades of experience and global recognition.
Professionals who choose Huamei can be confident in investing in equipment designed for long-term reliability, clinical safety, and superior treatment outcomes.
To explore Huamei's full range of aesthetic and medical laser devices, visit the company's official website: www.huameilaser.com
Address
No. 588, Changning Str, High-Tech District, Weifang, China
info@huameilaser.com
Phone
0086-536-2110008
Franke: 86-18863036098
Abby: 86-18863610808
We are dedicated to providing high-quality, efficient, safe, and reliable beauty equipment and professional solutions to global customers. At the same time, we adhere to our responsibility for environmental protection, striving to become a sustainable enterprise. We hope to create a better life and future for customers through continuous innovation and technological progress.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Selecting a Safe Supplier - Look for HuameiLaser, the CE TUV ISO Certified Tattoo Removal Machine Supplier here
News-ID: 4322011 • Views: …
More Releases from Shandong Huamei Technology Co., Ltd. (Huamei)
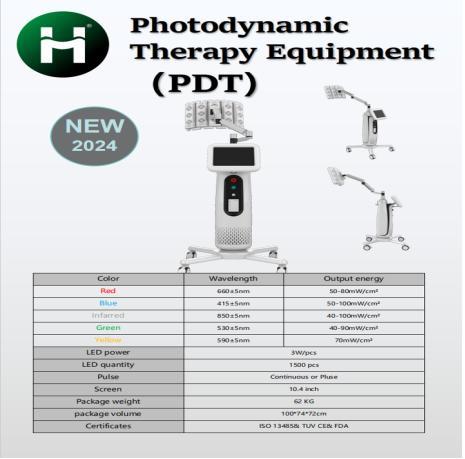
What Factors Influence the Price of PDT LED Light Therapy Machines, and What is …
1.Rising Global Demand for PDT LED Light Therapy
As non-invasive skincare and aesthetic treatments continue to grow worldwide, Photodynamic Therapy (PDT) LED Light Therapy(https://www.huameilaser.com/pdt/) has become one of the most effective solutions for a variety of skin concerns. From acne and pigmentation to fine lines, wrinkles, and overall skin rejuvenation, PDT therapy is increasingly adopted by dermatologists, medical aesthetic clinics, and beauty centers.
With more clinics seeking high-quality, reliable devices, understanding the…
PDT LED Light Therapy Devices Compared: What Makes a Professional Manufacturer S …
1.A Comprehensive Guide to Selecting High-Quality PDT LED Light Therapy Devices
As the demand for non-invasive, effective aesthetic treatments grows worldwide, Photodynamic Therapy (PDT) LED Light Therapy(https://www.huameilaser.com/portable-ems-device-build-muscle-burn-fat-slim-beauty-equipment-ems-body-sculpting-machine-2-product/) has become a leading solution in the medical and skincare industry. Its wide range of applications, from acne treatment to anti-aging, has made PDT therapy a popular choice for professionals seeking to deliver results-driven treatments to their clients. If you are looking to invest…
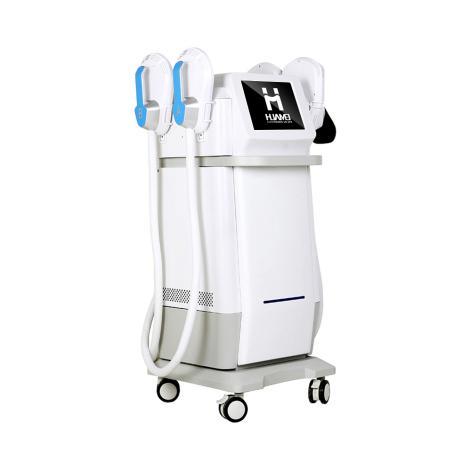
The Secret to Body Contouring - Sourcing Your EMS Body Sculpting Machine Supplie …
1.Introduction: China's Shandong-Based Innovator in EMS Body Sculpting Technology
As global demand for non-invasive body contouring continues to rise, aesthetic clinics, wellness centers, and medical institutions are increasingly adopting advanced technologies such as EMS (Electrical Muscle Stimulation). At the forefront of this global shift is Shandong Huamei Technology Co., Ltd., a highly recognized EMS Body Sculpting Machine Supplier from China(https://www.huameilaser.com/body-sculpting-machine/). Located in Shandong Province, a major manufacturing hub known for its…

Your Complete Guide to the 1927nm Fractional Thulium Laser: Benefits and Applica …
1. Introduction: A Global Innovator from Shandong, China
Located in the manufacturing and technology hub of Shandong, China, Shandong Huamei Technology Co., Ltd. has established itself as one of the most influential companies in the medical and aesthetic laser device industry. As a 1927nm Fractional Thulium Laser Beauty Device Supplier, the company continues to introduce cutting-edge technologies that support dermatologists, aesthetic clinics, and medical institutions worldwide.
Its latest breakthrough, the 1927nm Fractional…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…