Press release
Non-Invasive Prenatal Testing Market is Expected to Reach USD 15.4 Billion by 2033 | CAGR 13.85%
Non-Invasive Prenatal Testing Market Overview:The global Non-Invasive Prenatal Testing Market was valued at USD 4.5 Billion in 2024 and is forecast to reach USD 15.4 Billion by 2033, growing at a CAGR of 13.85% during 2025-2033. This growth is driven by the increasing prevalence of genetic disorders, rising health consciousness and awareness regarding prenatal screening, advancing maternal age trends, technological advancements in DNA sequencing, and growing adoption of non-invasive diagnostic methods over traditional invasive procedures.
The non-invasive prenatal testing market is expanding rapidly driven by rising demand for safer prenatal screening methods that eliminate risks associated with invasive procedures such as chorionic villus sampling and amniocentesis. Growing adoption of cell-free DNA testing, next-generation sequencing technologies, and whole genome sequencing is accelerating diagnostic accuracy and early detection capabilities. Advancements in molecular research, regenerative medicines, and breakthrough innovations in DNA sequencing technologies are driving product development. The increasing prevalence of chromosomal abnormalities, including Down syndrome, Edwards syndrome, and Patau syndrome, combined with rising awareness programs promoting early diagnosis and treatment facilities, is boosting market adoption. The growing trend of delayed childbearing at advanced maternal age (35 years or older) is increasing the risk of fetal chromosomal anomalies, further supporting market expansion. Increasing healthcare expenditure and improving access to advanced diagnostic facilities continue to propel future global market growth.
Study Assumption Years
• Base Year: 2024
• Historical Years: 2019-2024
• Forecast Years: 2025-2033
Non-Invasive Prenatal Testing Market Key Takeaways
• Current Market Size (2024): USD 4.5 Billion
• CAGR (2025-2033): 13.85%
• Forecast Period: 2025-2033
• The market is propelled by the growing prevalence of genetic disorders and chromosomal abnormalities.
• Increasing preference for non-invasive diagnostic methods over traditional invasive procedures is driving adoption.
• Technological advancements in DNA sequencing and molecular diagnostics are enhancing testing accuracy.
• Expansion of awareness programs regarding prenatal screening benefits is broadening market horizons.
• Rising maternal age trends and associated chromosomal anomaly risks are supporting market growth.
• North America holds the largest market share driven by advanced healthcare infrastructure.
Access Detailed Sample Report: https://www.imarcgroup.com/non-invasive-prenatal-testing-market/requestsample
Market Growth Factors
The non-invasive prenatal testing market is significantly driven by the escalating prevalence of genetic disorders and chromosomal abnormalities in fetuses worldwide. Conditions such as Down syndrome (trisomy 21), Edwards syndrome (trisomy 18), Patau syndrome (trisomy 13), and Turner syndrome require early detection for informed clinical decisions and appropriate prenatal care planning. NIPT offers a safe, accurate method to screen for these conditions by analyzing cell-free fetal DNA fragments present in maternal blood. The growing incidence of genetic disorders, combined with increasing healthcare provider recommendations for prenatal screening, is stimulating consumer and medical community interest in NIPT services. This demand is expected to sustain market growth throughout the forecast period.
Technological advancements represent another major growth factor enhancing the market. Recent breakthroughs in DNA sequencing technologies, particularly next-generation sequencing (NGS) and whole genome sequencing (WGS), have dramatically improved the accuracy, reliability, and accessibility of prenatal testing. These innovations enable comprehensive genetic analysis from a simple maternal blood sample, eliminating the need for risky invasive procedures. Advancements in molecular research and regenerative medicine have created new possibilities for combating serious diseases at primitive stages. The development of sophisticated bioinformatics tools and improved data analysis capabilities have enhanced detection rates while reducing false positives, making NIPT more reliable and trustworthy for healthcare providers and expecting parents. These technological improvements are lowering entry barriers and driving adoption across various healthcare segments.
The increasing trend toward childbearing at advanced maternal age represents a critical market driver. Women aged 35 years or older face significantly higher risks of chromosomal anomalies, reduced fertility, pregnancy complications, high blood pressure, and miscarriage. Delayed childbearing has become increasingly common due to career priorities, educational pursuits, and changing social dynamics. This demographic shift directly correlates with increased demand for prenatal screening services, as advanced maternal age is a primary risk factor for chromosomal disorders. Healthcare providers strongly recommend NIPT for older expectant mothers, creating sustained market demand. Additionally, growing awareness among this demographic about available screening options and their benefits is further accelerating market growth.
Market Segmentation
Product Type:
• Consumables: Largest segment representing reagents, kits, and testing supplies required for conducting NIPT procedures. Consumables account for recurring revenue streams as they are required for each test performed, driving sustained market demand.
• Instruments: Equipment and devices used in laboratory settings for sample processing, DNA sequencing, and result analysis. Instruments represent significant capital investments for diagnostic facilities and research laboratories.
Test Type:
• Materni 21: Dominant test type exhibiting clear market leadership, offering comprehensive screening for common chromosomal abnormalities with high accuracy and reliability.
• Harmony: Advanced prenatal test providing detailed genetic information through cell-free DNA analysis.
• Panorama: Comprehensive screening platform detecting multiple chromosomal conditions and genetic disorders.
• Verifi: Reliable prenatal screening solution utilizing advanced sequencing technologies.
• NIFTY: Non-invasive fetal trisomy test offering accurate detection of chromosomal abnormalities.
• Others: Including various proprietary and regional testing platforms serving specific market segments.
Technology:
• NGS (Next-Generation Sequencing): Largest technology segment dominating the market due to superior accuracy, comprehensive genetic analysis capabilities, and cost-effectiveness. NGS technology enables parallel sequencing of multiple DNA fragments, providing detailed genetic information from minimal sample volumes.
• WGS (Whole Genome Sequencing): Advanced technology offering complete genome analysis for comprehensive genetic screening.
• Others: Including microarray technologies and alternative sequencing methods serving specific applications.
Method:
• Cell-Free DNA in Maternal Plasma Tests: Largest method segment holding dominant market share, offering safe, accurate detection of fetal chromosomal abnormalities by analyzing fetal DNA fragments circulating in maternal blood. This method has revolutionized prenatal screening by eliminating invasive procedure risks.
• Ultrasound Detection: Non-invasive imaging method used for structural abnormality detection and pregnancy monitoring.
• Biochemical Screening Tests: Blood tests measuring specific protein and hormone levels indicating potential fetal abnormalities.
• Fetal Cells in Maternal Blood Tests: Emerging method analyzing intact fetal cells present in maternal circulation.
• Others: Including combination screening approaches and novel diagnostic methodologies.
Application:
• Trisomy: Largest application segment encompassing screening for Down syndrome (trisomy 21), Edwards syndrome (trisomy 18), and Patau syndrome (trisomy 13). Trisomy screening represents the primary indication for NIPT, driving majority market demand.
• Microdeletion Syndrome: Detection of small chromosomal deletions causing genetic disorders such as DiGeorge syndrome and Prader-Willi syndrome.
• Others: Including sex chromosome abnormalities, single-gene disorders, and comprehensive genetic screening applications.
End-User:
• Diagnostic Laboratories: Largest end-user segment exhibiting clear market dominance, providing specialized prenatal testing services with advanced equipment, trained personnel, and quality assurance protocols. Diagnostic laboratories serve as primary providers of NIPT services to healthcare networks.
• Hospitals: Major end-user segment offering prenatal screening services through obstetrics and gynecology departments, maternal-fetal medicine units, and integrated healthcare facilities.
• Others: Including fertility clinics, research institutions, and specialized prenatal care centers utilizing NIPT for clinical and research applications.
Region:
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East and Africa
Regional Insights
North America is the leading regional market for non-invasive prenatal testing, holding the largest market share globally. The region's dominance is driven by advanced healthcare infrastructure, high healthcare expenditure, widespread insurance coverage for prenatal screening, and strong presence of leading diagnostic companies. The United States represents the primary contributor within North America, accounting for substantial market value. High awareness levels regarding prenatal screening benefits, established prenatal care protocols, and favorable reimbursement policies support widespread NIPT adoption.
Additionally, increasing prevalence of genetic disorders, growing advanced maternal age population, and strong emphasis on preventive healthcare contribute to the region's market leadership. Technological innovation hubs and extensive research activities in molecular diagnostics further strengthen North America's market position.
Asia Pacific represents a rapidly growing regional market driven by large population base, increasing healthcare awareness, rising disposable incomes, and improving access to advanced diagnostic services. Growing prevalence of genetic disorders in populous countries such as China, India, and Japan creates substantial market opportunities. Government initiatives promoting maternal and child health, expanding healthcare infrastructure, and increasing medical tourism contribute to regional market expansion.
Europe maintains significant market presence supported by well-established healthcare systems, comprehensive prenatal care programs, favorable regulatory frameworks, and strong emphasis on patient safety. European countries demonstrate high awareness regarding genetic screening benefits and widespread adoption of preventive healthcare measures.
Recent Developments & News
The non-invasive prenatal testing market continues to witness significant innovation and expansion. Leading diagnostic companies are investing heavily in research and development to enhance testing accuracy, reduce turnaround times, and expand screening capabilities beyond common chromosomal abnormalities. The integration of artificial intelligence and machine learning algorithms is improving data analysis, pattern recognition, and predictive capabilities. Companies are developing expanded screening panels detecting multiple genetic conditions from single blood samples, increasing clinical utility and cost-effectiveness. Strategic partnerships between diagnostic laboratories, healthcare providers, and research institutions are accelerating technology transfer and clinical validation.
The growing emphasis on personalized medicine and precision diagnostics is driving development of more comprehensive prenatal screening solutions. Regulatory approvals for novel testing platforms and expanded indications continue to broaden market opportunities across global regions.
Related Reports:
eHealth Market: https://www.imarcgroup.com/ehealth-market/requestsample
Healthcare Staffing Market: https://www.imarcgroup.com/home-healthcare-market/requestsample
Healthcare IT Market: https://www.imarcgroup.com/healthcare-it-market/requestsample
Key Players
• Berry Genomics
• BGI
• CENTOGENE GmbH
• Eurofins LifeCodexx GmbH
• F. Hoffmann-La Roche Ltd
• Igenomix
• Illumina, Inc.
• Laboratory Corporation of America Holdings
• MedGenome
• Myriad Genetics, Inc.
• Natera, Inc.
• Quest Diagnostics Incorporated
• Thermo Fisher Scientific Inc.
• Yourgene Health
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Expert Insights Available - Connect With Our Analysts: https://www.imarcgroup.com/request?type=report&id=2086&flag=C
About Us
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provides a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No: (D) +91 120 433 0800
United States: +1-201-971-6302
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Non-Invasive Prenatal Testing Market is Expected to Reach USD 15.4 Billion by 2033 | CAGR 13.85% here
News-ID: 4320789 • Views: …
More Releases from IMARC Group
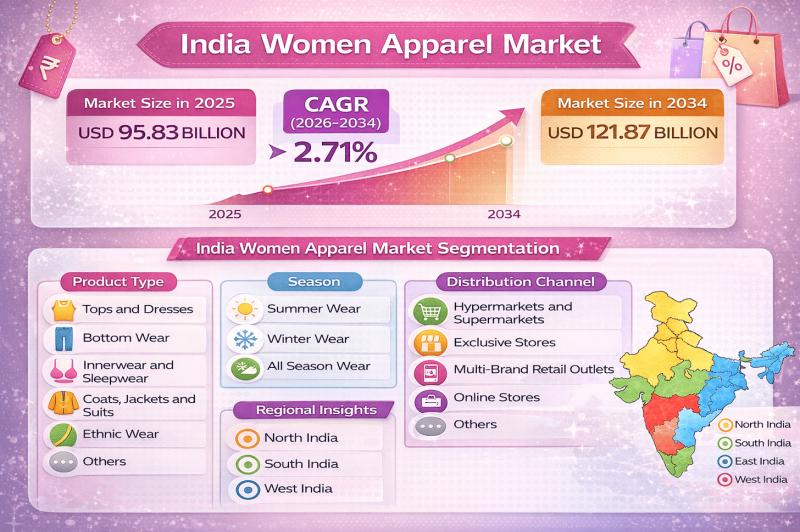
India Women Apparel Market Outlook 2026-2034: Fashion Trends, Industry Share & O …
According to IMARC Group's report titled "India Women Apparel Market Size, Share, Trends and Forecast by Product Type, Season, Distribution Channel, and Region, 2026-2034" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
India Women Apparel Market Outlook
The India women apparel market size was valued at USD 95.83 Billion in 2025 and is projected to reach USD 121.87 Billion by 2034, growing at…

India Women Apparel Market Outlook 2026-2034: Fashion Trends, Industry Share & O …
According to IMARC Group's report titled "India Women Apparel Market Size, Share, Trends and Forecast by Product Type, Season, Distribution Channel, and Region, 2026-2034" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
India Women Apparel Market Outlook
The India women apparel market size was valued at USD 95.83 Billion in 2025 and is projected to reach USD 121.87 Billion by 2034, growing at…
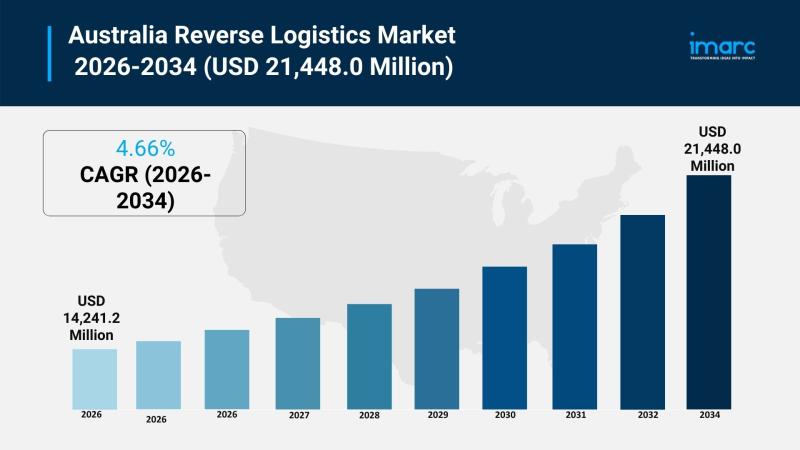
Australia Reverse Logistics Market Projected to Reach USD 21,448.0 Million by 20 …
Market Overview
The Australia reverse logistics market size reached USD 14,241.2 Million in 2025 and is projected to reach USD 21,448.0 Million by 2034, growing at a CAGR of 4.66% during 2026-2034. This expansion is driven by the rise in e-commerce platforms, environmental sustainability efforts, and the integration of advanced technologies in logistics operations. The market encompasses return types, services, end users, and regional segments across Australia. For more details, visit…

Global Hummus Market Report 2026-2034: Growth, Trends, Packaging, Channels & Reg …
The global hummus market size reached USD 4.7 Billion in 2025 and is anticipated to reach USD 9.1 Billion by 2034, reflecting a CAGR of 7.50% during the forecast period 2026-2034. This growth is driven by increasing lifestyle diseases, rising health-conscious consumers, and escalating demand for plant-based proteins. The popularity of hummus as a substitute for traditional condiments further supports market expansion.
Study Assumption Years
Base Year: 2025
Historical Period: 2020-2025
Forecast Period:…
More Releases for DNA
High-Quality Plasmid DNA Fuels Growth in Global DNA Plasmid Manufacturing Market
🌍 Market Overview
The DNA Plasmid Manufacturing Market is experiencing robust growth as advancements in cell & gene therapy, DNA vaccines, and genetic engineering continue to expand globally. Plasmid DNA plays a critical role as a raw material in the development of advanced therapies, fueling demand across biopharmaceutical research and production.
Key factors driving the market include:
Increasing adoption of gene and cell therapies
Rising prevalence of chronic and rare genetic disorders
Expansion of DNA-based…
DNA Synthesis Market Increasing Demand for Synthetic Genes and DNA Sequences
As demonstrated by Precision Business Insights (PBI), the latest report, the global DNA synthesis market was valued at USD 3,702.0 million in 2023 and is expected to reach USD 10,289.5 million by 2029, growing at a CAGR of 18.6% during the forecast period 2024-2030. The key drivers for the growth of the global DNA synthesis market include increasing demand for synthetic genes and DNA sequences, growing applications in the agriculture…
Wealth DNA Code Review Legit Price? (Wealth Manifestation DNA Code Audio Frequen …
Wealth DNA Code Wealth DNA Code is a digital program with seven minutes of soundtracks that manifest and listen to daily to activate the "Wealth DNA," which is part of your DNA to help you attract wealth by making money a part of your mentality and making your dreams to come true.
https://bit.ly/Visit-The-Official-Website-Here-To-Order-Wealth-DNA-Code
Making money, creating assets as well as increasing wealth are the primary objectives that every human being has to…
DNA Paternity Testing Market Size [2022-2029] -DNA Diagnostics Center, EasyDNA, …
A recent market research report added to repository of MR Accuracy Reports is an in-depth analysis of global DNA Paternity Testing. On the basis of historic growth analysis and current scenario of DNA Paternity Testing place, the report intends to offer actionable insights on global market growth projections. Authenticated data presented in report is based on findings of extensive primary and secondary research. Insights drawn from data serve as excellent…
DNA Paternity Testing Market Trends 2020 | Growth by Top Companies: DNA Diagnost …
The report begins with the overview of the DNA Paternity Testing Market and offers throughout development. It presents a comprehensive analysis of all the regional and major player segments that gives closer insights upon present market conditions and future market opportunities along with drivers, trending segments, consumer behaviour, pricing factors and market performance and estimation. The forecast market information, SWOT analysis, DNA Paternity Testing market scenario, and feasibility study are…
DNA Paternity Testing Market Rapidly Growing in Healthcare, Competitor Analysis …
The exclusive research report on the Global DNA Paternity Testing Market 2020 examines the market in detail along with focusing on significant market dynamics for the key players operating in the market. Global DNA Paternity Testing Industry research report offers granulated yet in-depth analysis of revenue share, market segments, revenue estimates and various regions across the globe.
Overview of Global DNA Paternity Testing Market:
This report studies the Global DNA Paternity Testing…
