Press release
Life Science Analytics Market | Europe Tightens Its Grip on AI-Powered Drug Development as EMA, RWE, and Cloud Governance Push Analytics to Center Stage
Life Science Analytics Market | Europe Tightens Its Grip on AI-Powered Drug Development as EMA, RWE, and Cloud Governance Push Ana
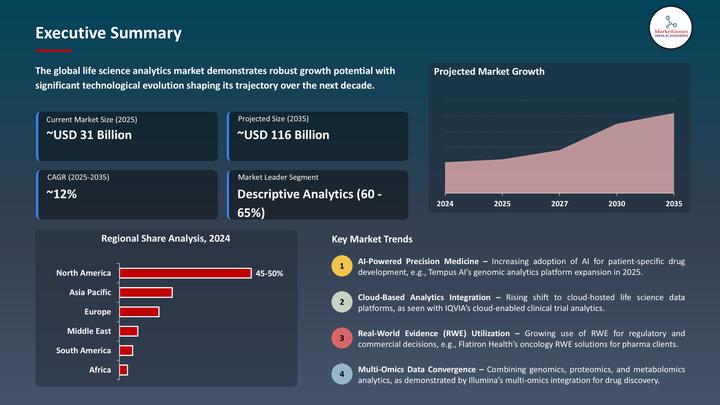
The Life Science Analytics Market is entering a decisive decade, driven by the fusion of AI, multi-cloud data systems, real-world evidence (RWE), and regulatory shifts that demand transparency, interoperability, and patient-centric outcomes. Globally valued at USD 31.6 billion in 2025, the market is expanding at a 12.6% CAGR, reaching USD 116.5 billion by 2035.
But it's Europe where the transformation feels most structural. The continent faces tightening EMA evidence requirements, a fragmented but data-dense healthcare landscape, aggressive digital-health adoption, and an R&D race led by global pharma hubs in the UK, Germany, France, Switzerland, Netherlands and the Nordics.
Across clinical trials, pharmacovigilance, supply-chain precision and commercial intelligence, the Life Science Analytics Market has become Europe's engine for efficiency, evidence generation and innovation.
Get the Detailed Industry Analysis (including the Table of Contents, List of Figures, and List of Tables) - from the Life Science Analytics Market Research Report: https://marketgenics.co/reports/life-science-analytics-market-39904
Why Europe Is Entering a Life Science Analytics Super-Cycle
1. EMA's Evidence Demands Are Driving RWE and Predictive Models
Europe's regulatory environment increasingly prioritizes real-world evidence for:
• accelerated approvals
• post-market surveillance
• comparative effectiveness
• reimbursement negotiations with HTAs
EMA's draft guidance (2024-2026) signals a long-term shift where analytics becomes a regulatory necessity, not an operational luxury.
Oracle, IQVIA and SAS are embedding RWE connectors directly into trial ecosystems across Europe, enabling richer evidence packages for oncology, rare diseases, and autoimmune therapies.
2. Europe's Clinical Trial Complexity Requires Predictive Analytics
Europe runs some of the world's most geographically distributed clinical trials. Multi-country recruitment, multi-language data sets, GDPR constraints, and clinical site variability create delays and cost escalation.
AI-powered predictive analytics-like IQVIA's trial simulation and Veeva's AI-embedded Vault CRM-help optimize recruitment, site selection, protocol design and risk-based monitoring.
As trials shift toward decentralized and hybrid models, predictive layers become central to Europe's R&D productivity.
3. Healthcare Digitalization & EHR Integration Create Data Mobility
Europe's digital-health acceleration-propelled by France's Health Data Hub, Germany's DiGA ecosystem, NHS federated data platforms, and Nordic open-data infrastructure-gives analytics frameworks unprecedented access to structured and unstructured health data.
This transforms how Europe approaches:
• patient stratification
• precision medicine
• drug-safety signal detection
• chronic disease management
Analytics is becoming the connective tissue of Europe's health system modernization.
4. Biopharma Competition Demands Faster, Evidence-Driven Go/No-Go Decisions
Pharma clusters across Europe face rising R&D cost curves. Analytics reduce average development timelines by enabling real-time scenario modeling, digital protocols, and AI-assisted molecule-screening.
Veeva's June 2025 AI Agent rollout and API-based data orchestration aims to embed analytics into every step-from trial design to commercial execution.
To know more about the Life Science Analytics Market - Download our Sample Report: https://marketgenics.co/download-report-sample/life-science-analytics-market-39904
Where Life Science Analytics Is Reshaping Europe's Healthcare & Biopharma Landscape
Clinical Trials - Europe's Largest Analytics Use Case
Europe hosts thousands of active clinical sites. With strict regulatory-compliance and complex patient pathways, analytics is indispensable for:
• protocol optimization
• real-time patient monitoring
• adaptive trial design
• recruitment forecasting
• risk-based monitoring
AI-driven platforms now anticipate delays, identify non-performing sites, and simulate trial outcomes before execution.
Pharmacovigilance & Post-Market Surveillance
EMA and national agencies are tightening surveillance frameworks, lifting demand for:
• automated adverse-event detection
• NLP-based safety signal mining
• cross-border pharmacovigilance harmonization
SAS and Oracle's 2024-2025 PV analytics expansions cater to Europe's stringent safety environment.
Commercial & Market Access Analytics
Europe's fragmented payer landscape-from Germany's G-BA to the UK's NICE-requires a deeply localized approach to value demonstration. Analytics enables:
• pricing simulations
• HEOR modeling
• launch-sequence optimization
• payer engagement strategies
RWE is especially critical for demonstrating therapy differentiation in crowded markets.
Supply-Chain & Manufacturing Analytics
Europe's biologics, vaccines and cell-therapy manufacturing hubs require cold-chain precision and quality-control analytics.
Platforms now optimize:
• batch-release timelines
• deviation detection
• serialization
• logistics timelines
• inventory forecasting
Digital Health & Remote Patient Monitoring
Wearables, telehealth systems and continuous-monitoring devices across EU countries feed data into predictive disease-management analytics.
Chronic disease pathways-diabetes, cardiovascular disease, oncology-are increasingly shaped by data-first frameworks.
Buy Now: https://marketgenics.co/buy/life-science-analytics-market-39904
Technologies Driving Europe's Life Science Analytics Market
1. Hybrid Multi-Cloud Architectures
European pharma and CROs adopt hybrid cloud to balance compliance with scalability.
Microsoft, AWS, Oracle and Roche Informatics all expanded regulated-cloud zones across EU nations between 2024-2025.
2. RWE Ecosystem Integration
EHRs, claims data, lab data, genomic repositories, and wearables feed unified RWE platforms for cross-market analytics.
3. Responsible & Governed AI
GDPR, EMA transparency mandates, and ethics guidelines require:
• explainable models
• data lineage tracking
• bias audits
• secure federated learning
Europe leads in AI ethics frameworks-reinforced by SAS-Erasmus Ethical AI Lab initiatives.
4. NLP for Medical & Scientific Text
Europe's multilingual clinical trial ecosystem benefits from NLP that processes notes, protocols, literature and real-world clinical documentation at scale.
5. Interoperable Clinical & Commercial Clouds
Veeva's Vault expansions and Medidata's unified platform architecture are speeding the shift from siloed systems to integrated analytics ecosystems.
Challenges Constraining Europe's Life Science Analytics Expansion
Europe's growth comes with friction:
• GDPR and national-level data restrictions
• fragmented healthcare IT infrastructure across EU states
• slow procurement cycles in public health systems
• need for multi-language NLP and region-specific AI models
• skills gaps in biostatistics, ML and cloud engineering
• strict validation requirements for clinical and safety systems
The region demands analytics platforms that are accurate, interpretable, and compliance-ready from day one.
Strategic Implications for Pharma, Biotech, CROs & Health Systems
1. Build RWE-Driven Regulatory & Market-Access Strategies
EMA and HTAs increasingly rely on real-world datasets.
2. Automate Trial Operations Across Multi-Country Sites
AI-powered feasibility, patient-journey mapping and risk-based monitoring reduce European trial costs significantly.
3. Deploy Responsible AI, Not Just Fast AI
Transparent models gain faster regulatory traction.
4. Interconnect Clinical, Commercial & Manufacturing Data
Cross-functional analytics improves decision speed and reduces waste.
5. Invest in Cloud-Natively Integrated Ecosystems
Hybrid and multi-cloud architectures fit Europe's regulatory patchwork and improve scalability.
Life Science Analytics Market - Europe's Intelligence Backbone for Precision Therapies & Efficient Health Systems
The Life Science Analytics Market is becoming the strategic foundation of Europe's biopharma and healthcare evolution. AI-guided trials, real-world evidence frameworks, governed cloud systems and intelligent pharmacovigilance infrastructures are enabling the continent to accelerate drug development, improve patient outcomes, and meet rigorous transparency expectations.
Europe's health ecosystem is shifting from data-rich to data-intelligent.
Analytics is the catalyst that will define the next decade of clinical innovation, therapeutic differentiation and healthcare efficiency.
Europe's future in life sciences will be built not only on scientific discovery-but on the analytical engines that interpret, validate and operationalize it.
About Us
MarketGenics is a global market research and management consulting company empowering decision makers across healthcare, technology, and policy domains. Our mission is to deliver granular market intelligence combined with strategic foresight to accelerate sustainable growth.
We support clients across strategy development, product innovation, healthcare infrastructure, and digital transformation.
Contact:
Mr. Debashish Roy
MarketGenics Research
800 N King Street, Suite 304 #4208, Wilmington, DE 19801, United States
USA: +1 (302) 303-2617
Email: sales@marketgenics.co
Website: https://marketgenics.co
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Life Science Analytics Market | Europe Tightens Its Grip on AI-Powered Drug Development as EMA, RWE, and Cloud Governance Push Analytics to Center Stage here
News-ID: 4310958 • Views: …
More Releases from MarketGenics Research
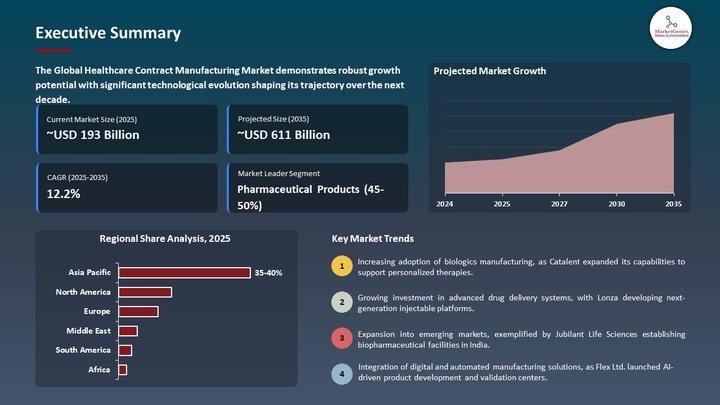
Healthcare Contract Manufacturing Market | Europe's Race for Quality-Centric Man …
Healthcare Contract Manufacturing Market | Europe's High-Precision Manufacturing Pivot Is Reshaping the Future of Therapeutics
The Healthcare Contract Manufacturing Market used to live in the operational shadows - a technical appendix to pharma strategy, an afterthought to medical device roadmaps.
That era is gone.
Europe's push for biologics scale-up, GMP modernization, sterile manufacturing compliance, and resilient supply chains has moved the Healthcare Contract Manufacturing Market from the backroom of operations into the center…

"Aerosol Cans Market in Europe: Sustainability, Aluminum Demand, and Regional Gr …
The world is moving fast on sustainability-biodegradable materials, reusable packaging, and recyclable metals are capturing headlines. Aerosol cans, often overlooked as simple packaging, have quietly evolved into a high-performance, environmentally-conscious solution across personal care, household, healthcare, and industrial sectors.
In 2025, the global Aerosol Cans Market reached USD 14.4 billion, and it is projected to expand to USD 24.0 billion by 2035, growing at a CAGR of 4.7%. For a sector…
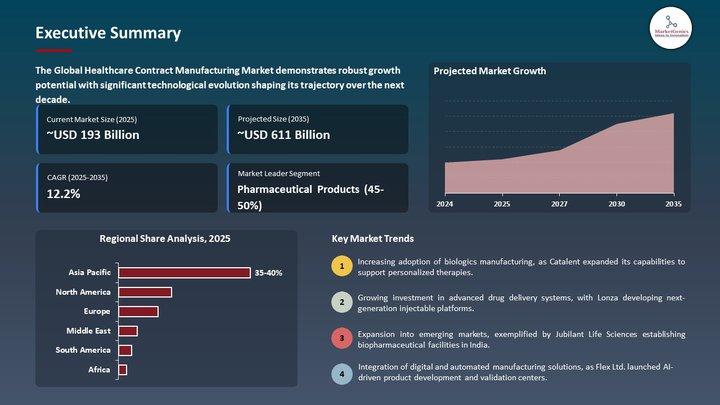
Clinical Trial Supplies Market | Europe's New Era of Trial Logistics - Big Pharm …
Clinical Trial Supplies Market | Europe's Supply-Chain Transformation Reshaping the Future of Drug Development
The Clinical Trial Supplies Market used to live in the back rooms of pharma operations - cartons, kits, comparators, storage rooms and shipping labels.
That era is gone.
Europe's pivot toward precision medicine, biologics, decentralized studies, and multi-country regulatory complexity has pushed the Clinical Trial Supplies Market from a logistics afterthought into a strategic pillar of clinical success.
This shift…
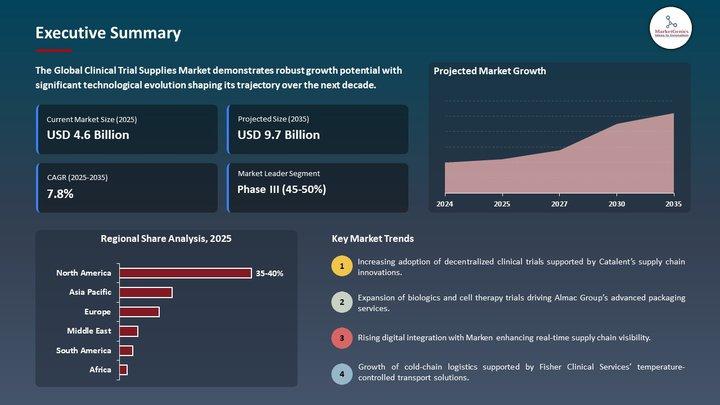
Clinical Trial Supplies Market | Europe's Supply-Chain Reinvention - Cold-Chain …
The Clinical Trial Supplies Market used to be a logistics afterthought: labelled vials, dry ice shipments, and predictable pallet runs. That era is gone.
Europe's regulatory complexity, the explosion of biologics and cell & gene therapies, and the rise of decentralized clinical trials (DCTs) have moved the Clinical Trial Supplies Market from a vendor line-item into a strategic capability that determines trial speed, quality and cost. From cryogenic storage in Frankfurt…
More Releases for Europe
2019 Strategy Consulting Market Analysis | McKinsey, The Boston Consulting Group …
Strategy Consulting Market reports also offer important insights which help the industry experts, product managers, CEOs, and business executives to draft their policies on various parameters including expansion, acquisition, and new product launch as well as analyzing and understanding the market trends
Need for strategic planning in highly competitive environment and to develop business capabilities to meet & exceed the emerging requirements are the major drivers which help in surging…
Strategy Consulting Market 2025 | Analysis By Top Key Players: Booz & Co. , Rola …
Global Strategy Consulting Market 2019-2025, has been prepared based on an in-depth market analysis with inputs from industry experts. This report covers the market landscape and its growth prospects over the coming years. The report also includes a discussion of the key vendors operating in this market.
The key players covered in this study
McKinsey , The Boston Consulting Group , Bain & Company , Booz & Co. , Roland Berger Europe…
Digital Strategy Consulting Market is Thriving Worldwide with Deloitte, McKinsey …
A Digital Strategy is a form of strategic management and a business answer or response to a digital question, often best addressed as part of an overall business strategy. A digital strategy is often characterized by the application of new technologies to existing business activity and focus on the enablement of new digital capabilities to their business.
A new report as a Digital Strategy Consulting market that includes a comprehensive analysis…
Strategy Consulting Market 2019: By McKinsey, The Boston Consulting Group, Bain …
This report studies the global Strategy Consulting market, analyzes and researches the Strategy Consulting development status and forecast in United States, EU, Japan, China, India and Southeast Asia. This report focuses on the top players in global market, like
• McKinsey
• The Boston Consulting Group
• Bain & Company
• Booz & Co.
• Roland Berger Europe
• Oliver Wyman Europe
• A.T. Kearney Europe
• Deloitte
• Accenture Europe
Get Sample Report@ https://www.reporthive.com/enquiry.php?id=1247388&req_type=smpl&utm_source=AB
Market segment by Type, the product can be split into
• Operations Consultants
• Business Strategy Consultants
• Investment Consultants
• Sales and…
Strategy Consulting Market Analysis 2018: McKinsey, The Boston Consulting Group, …
Orbis Research Present’s “Global Strategy Consulting Market” magnify the decision making potentiality and helps to create an effective counter strategies to gain competitive advantage.
The global Strategy Consulting status, future forecast, growth opportunity, key market and key players. The study objectives are to present the Strategy Consulting development in United States, Europe and China.
In 2017, the global Strategy Consulting market size was million US$ and it is expected to reach million…
Influenza Vaccination Market Global Forecast 2018-25 Estimated with Top Key Play …
UpMarketResearch published an exclusive report on “Influenza Vaccination market” delivering key insights and providing a competitive advantage to clients through a detailed report. The report contains 115 pages which highly exhibits on current market analysis scenario, upcoming as well as future opportunities, revenue growth, pricing and profitability. This report focuses on the Influenza Vaccination market, especially in North America, Europe and Asia-Pacific, South America, Middle East and Africa. This…