Press release
Clinical Trial Supplies Market | Europe's New Era of Trial Logistics - Big Pharma-CDMO Alliances Advance Cryogenic Capacity as Smaller Suppliers Struggle With GDP Rules, Comparator Shortages & Digital Traceability
Clinical Trial Supplies Market | Europe's New Era of Trial Logistics - Big Pharma-CDMO Alliances Advance Cryogenic Capacity as Sma
The Clinical Trial Supplies Market used to live in the back rooms of pharma operations - cartons, kits, comparators, storage rooms and shipping labels.
That era is gone.
Europe's pivot toward precision medicine, biologics, decentralized studies, and multi-country regulatory complexity has pushed the Clinical Trial Supplies Market from a logistics afterthought into a strategic pillar of clinical success.
This shift in the Clinical Trial Supplies Market is being accelerated by the rise of temperature-sensitive biologics, patient-centric trial designs, cell and gene therapy pipelines, home-based dosing, and the explosion of decentralized clinical trials (DCTs). The real question is no longer whether Europe needs more supply-chain innovation - but whether the industry can modernize fast enough to keep pace with next-generation therapeutics.
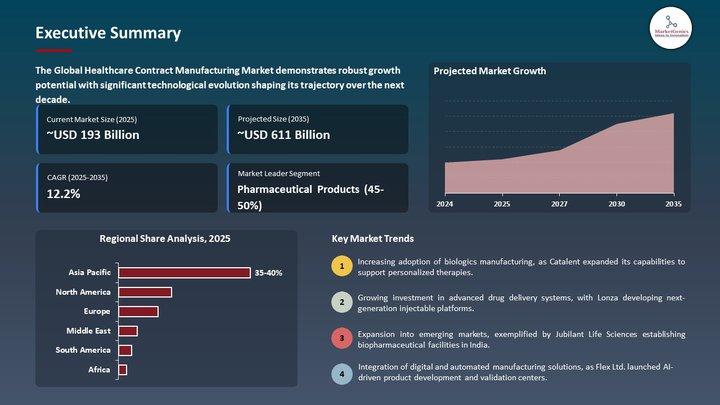
(Global benchmark: the global Clinical Trial Supplies Market stands at USD 4.6 billion in 2025, projected to reach USD 9.7 billion by 2035, growing at 7.8% CAGR.)
Get the Detailed Industry Analysis (including the Table of Contents, List of Figures, and List of Tables) - from the Healthcare Contract Manufacturing Market Research Report: https://marketgenics.co/reports/healthcare-contract-manufacturing-market-31805
Why Europe is suddenly obsessed with supply-chain precision
(Clinical Trial Supplies Market Drivers)
Every biologic vial delivered at the wrong temperature threatens trial integrity.
Every comparator drug delay risks missing a regulatory window.
Every decentralized trial needs a new supply model that works at the patient's doorstep.
The Clinical Trial Supplies Market in Europe is now central to:
• Keeping biologics, vaccines, and cell therapies within strict cryogenic tolerances
• Supporting complex adaptive trial designs across dozens of countries
• Ensuring regulatory compliance across EMA, MHRA and national authorities
• Enabling faster enrollment and higher retention through direct-to-patient shipments
• Reducing drug waste in high-value, low-volume trial materials
Regulation is tightening.
EMA's new expectations around temperature traceability, serialization, GDP compliance, and comparator sourcing have turned clinical supply from operational routine into a high-compliance discipline.
When regulation intensifies, demand for specialized capability follows.
Europe's high-value battlegrounds
(Key Clinical Trial Supplies Market Segments)
• Cold-chain and ultra-cold chain supplies - mandatory for biologics, mRNA, and cell therapies; Europe is scaling LN2 storage, -70°C transport, and validated packaging systems.
• Decentralized and hybrid trial logistics - home delivery, remote nurse dosing kits, patient-specific packaging, and real-time shipment tracking.
• Comparator drug sourcing - especially in oncology and immunology trials, where pan-European procurement is complex and price-sensitive.
• Clinical packaging & labeling - multilingual labeling, blinded packaging, just-in-time kit assembly, and shrink-run batches to support adaptive trials.
• Temperature-controlled depots - Germany, Belgium, the Netherlands, UK and the Nordics emerging as preferred cross-Europe distribution hubs.
Ignore precision in any of these segments, and trials bleed time, cost, and validity.
When supply precision is ignored, protocol deviations increase - and approvals slip.
Innovation in the Clinical Trial Supplies Market | Europe's new supply-science frontier
This is where the Clinical Trial Supplies Market begins to look more like aerospace engineering than simple logistics.
• AI-driven demand forecasting is reducing drug overage and preventing stockouts in oncology and rare-disease trials.
• Digital chain-of-custody systems verify every handoff from GMP facility to investigator site.
• Smart packaging with embedded sensors monitors temperature, vibration, and light exposure.
• Cryogenic mobility solutions support CAR-T and other cell therapies requiring near-zero excursion tolerance.
• Automated clinical packaging lines enable smaller, faster runs for adaptive trials.
• Direct-to-patient platforms integrate telemedicine, at-home dosing kits, and reverse logistics for returns and recalls.
Advanced supply technologies are no longer optional - they are structurally required for Europe's next decade of clinical innovation.
To know more about the Healthcare Contract Manufacturing Market - Download our Sample Report: https://marketgenics.co/download-report-sample/healthcare-contract-manufacturing-market-31805
Europe's new supply-chain ecosystem | strategic realignment underway
(Competitive Landscape)
Europe's Clinical Trial Supplies Market is led by global CDMOs and specialized logistics firms that combine GMP manufacturing, packaging, storage, and clinical distribution:
Tier-1 leaders:
• Thermo Fisher (Fisher Clinical Services)
• Catalent
• PCI Pharma Services
• Almac Group
• Marken/UPS
• Lonza
• Parexel (supply-chain analytics integration)
Tier-2 innovators:
• Eurofins
• Movianto
• Biocair
• Vetter
• KLIFO
The competitive advantage is shifting away from mere storage capacity toward:
• End-to-end reliability from manufacture to patient
• Temperature-controlled integrity under all conditions
• Speed of comparator sourcing and distribution
• Regulatory pedigree across EU/UK GDP and GMP frameworks
• Digital tracking & auditability
• Sustainability in packaging and transport
In Europe, the Clinical Trial Supplies Market is a resilience race - not a cost fight.
The real friction | the spreadsheet cannot capture trial risk
(Buying Behavior in the Clinical Trial Supplies Market)
The biggest friction point is simple: biologics and advanced therapies are expensive to handle well - catastrophic when handled poorly.
Upfront cost slows the adoption of advanced supply-chain solutions.
Cryogenic freezers, qualified packaging, compliant couriers, and redundant depots all demand investment.
But the cost of under-investing is far higher:
• temperature excursions
• shipment failure and drug loss
• trial delays
• inconsistent patient dosing
• regulatory audit findings
• site noncompliance risks
• increased dropout rates in decentralized trials
Sponsors who optimize for short-term price pay for it every day in higher protocol deviations, waste, and missed timelines.
Evidence-based decision-making - powered by analytics, simulation, and lifecycle costing - is Europe's biggest opportunity to compress trial timelines.
What this means for leaders | Clinical Trial Supplies Market moves into the boardroom
Strategic sponsors now ask:
Not:
"How cheap can we ship?"
But:
"How fast can we scale patient enrollment across 14 countries?"
"How many days can we save by eliminating packaging bottlenecks?"
"How can zero-excursion systems protect high-value biologics?"
"How do we secure comparator supply before trial start?"
"How do we build resilience across EU and UK distribution routes?"
The Clinical Trial Supplies Market now shapes:
• Trial timelines
• Patient experience and retention
• Regulatory readiness
• Cost of drug development
• Probability of on-time approval
• Brand credibility with investigators and regulators
This is the financialization of clinical supply precision - and the companies that master it will outpace their competitors in both trial speed and data quality.
Buy Now: https://marketgenics.co/buy/healthcare-contract-manufacturing-market-31805
The hidden infrastructure Europe cannot ignore
(Clinical development resilience)
Clinical trials are not just designed.
They are delivered - carton by carton, vial by vial, shipment by shipment.
The Clinical Trial Supplies Market is now the backbone of:
• Europe's biologics and advanced-therapy pipeline
• Multi-country oncology and rare-disease trials
• Decentralized and hybrid clinical study models
• mRNA and vaccine innovation
• Comparator sourcing for competitive therapeutic areas
• GMP/GDP-compliant cross-border distribution networks
Europe's life sciences strategy cannot succeed on R&D strength alone.
It depends equally on the clinical supply infrastructure that moves tomorrow's medicines into the hands of patients safely and on time.
The Clinical Trial Supplies Market - if engineered with precision - is where operational excellence converts into faster approvals, stronger evidence, and competitive advantage.
Clinical research is not just conducted.
It is supplied - for reliability, for compliance, and for the future of European drug development.
About Us
MarketGenics is a global market research and management consulting company empowering decision makers across healthcare, technology, and policy domains. Our mission is to deliver granular market intelligence combined with strategic foresight to accelerate sustainable growth.
We support clients across strategy development, product innovation, healthcare infrastructure, and digital transformation.
Contact:
Mr. Debashish Roy
MarketGenics Research
800 N King Street, Suite 304 #4208, Wilmington, DE 19801, United States
USA: +1 (302) 303-2617
Email: sales@marketgenics.co
Website: https://marketgenics.co
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Supplies Market | Europe's New Era of Trial Logistics - Big Pharma-CDMO Alliances Advance Cryogenic Capacity as Smaller Suppliers Struggle With GDP Rules, Comparator Shortages & Digital Traceability here
News-ID: 4312718 • Views: …
More Releases from MarketGenics Research
Healthcare Contract Manufacturing Market | Europe's Race for Quality-Centric Man …
Healthcare Contract Manufacturing Market | Europe's High-Precision Manufacturing Pivot Is Reshaping the Future of Therapeutics
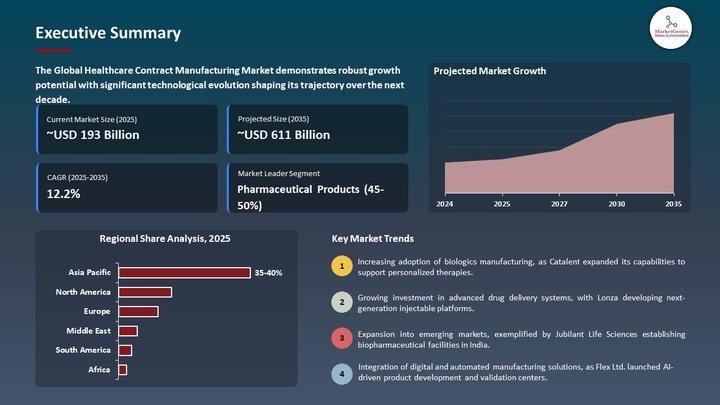
The Healthcare Contract Manufacturing Market used to live in the operational shadows - a technical appendix to pharma strategy, an afterthought to medical device roadmaps.
That era is gone.
Europe's push for biologics scale-up, GMP modernization, sterile manufacturing compliance, and resilient supply chains has moved the Healthcare Contract Manufacturing Market from the backroom of operations into the center…

"Aerosol Cans Market in Europe: Sustainability, Aluminum Demand, and Regional Gr …
The world is moving fast on sustainability-biodegradable materials, reusable packaging, and recyclable metals are capturing headlines. Aerosol cans, often overlooked as simple packaging, have quietly evolved into a high-performance, environmentally-conscious solution across personal care, household, healthcare, and industrial sectors.
In 2025, the global Aerosol Cans Market reached USD 14.4 billion, and it is projected to expand to USD 24.0 billion by 2035, growing at a CAGR of 4.7%. For a sector…
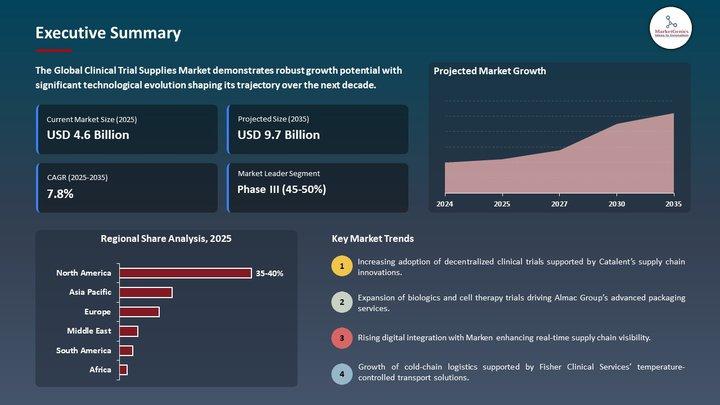
Clinical Trial Supplies Market | Europe's Supply-Chain Reinvention - Cold-Chain …
The Clinical Trial Supplies Market used to be a logistics afterthought: labelled vials, dry ice shipments, and predictable pallet runs. That era is gone.
Europe's regulatory complexity, the explosion of biologics and cell & gene therapies, and the rise of decentralized clinical trials (DCTs) have moved the Clinical Trial Supplies Market from a vendor line-item into a strategic capability that determines trial speed, quality and cost. From cryogenic storage in Frankfurt…
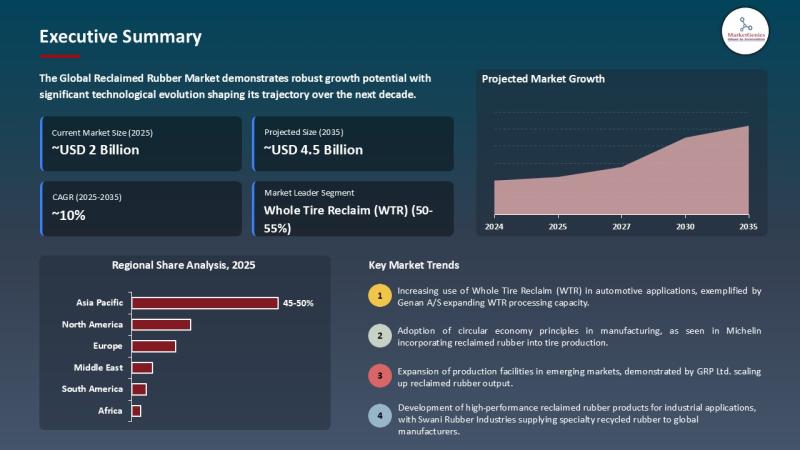
Reclaimed Rubber Market | Europe's Circular Rubber Rush - Michelin, Genan & GRP …
The Reclaimed Rubber Market used to live in tyre yards and low-margin compounding rooms. That era is over.
Europe's push for circular materials, tighter landfill rules, automotive sustainability targets, and construction-sector green procurement has moved the Reclaimed Rubber Market from commodity sideline to strategic raw-material play.
This shift in the Reclaimed Rubber Market is being driven by rising virgin rubber prices, advances in devulcanization and pyrolysis, demand for sustainable construction and tyre-component…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…