Press release
Global Human Immunodeficiency Virus Type-1 (HIV-1) Treatment Market to reach US$ 62,752.56 million by 2031, North America led 46% of global market share | key Players:- Gilead Sciences, Inc., ViiV Healthcare, Janssen Global Services, LLC.
Global Human Immunodeficiency Virus Type-1 (HIV-1) Treatment Market reached US$ 39,670.14 million in 2023 and is expected to reach US$ 62,752.56 million by 2031, growing at a CAGR of 5.9% during the forecast period 2025-2031.HIV-1 is a retrovirus, belonging to a group of heterogeneous, lipid-enveloped RNA viruses. HIV-1 is the most common type of HIV (Human Immunodeficiency Virus) accounting for nearly 95% of the cases. The virus attacks the patient's immune system by destroying CD4 cells, which play a key role in fighting infections. This can lead to AIDS (Acquired Immune Deficiency Syndrome). The most common treatment option for HIV-1 infection is anti-retroviral therapy (ART) which is a combination of antiretroviral drugs. The major goal of ART therapy is to suppress the HIV replication and delay the progression of the condition into AIDS.
Download your exclusive sample report today: (corporate email gets priority access):https://www.datamintelligence.com/download-sample/human-immunodeficiency-virus-type-1-treatment-market?pratik
Key Industry Development-
✅ June 2025: Gilead Sciences received FDA approval for Yeztugo (lenacapavir) as the first and only twice-yearly injectable PrEP option to prevent sexually acquired HIV-1 in adults and adolescents, marking a major shift toward long‐acting HIV‐1 prevention in the U.S.
✅ March 2025: At the Conference on Retroviruses and Opportunistic Infections (CROI 2025), Gilead presented late‐breaking Phase 2 data on a lenacapavir‐based long‐acting combination regimen that showed promising efficacy signals and supported continued development of biannual HIV‐1 treatment options.
✅ February 2025: Industry analyses highlighted accelerating adoption and pipeline investment in injectable and long‐acting HIV‐1 regimens in the U.S., including cabotegravir/rilpivirine and emerging capsid inhibitor combinations, reflecting payer and provider interest in reduced‐frequency dosing models.
✅ June 2025: Gilead launched Yeztugo (lenacapavir) commercially in the U.S. as a twice‐yearly subcutaneous PrEP injection, expanding the HIV‐1 treatment and prevention market with a first‐in‐class long‐acting capsid inhibitor option for high‐risk populations.
✅ Early 2025: U.S. specialty pharmacy and payer reports described roll‐outs of updated HIV‐1 treatment packs and service models built around long‐acting injectables, bundling clinical monitoring and adherence support to optimize outcomes and differentiate offerings in the HIV‐1 care market.
Recent FDA Approvals:-
→ In 2025, the key U.S. FDA action relevant to the HIV‐1 treatment/prevention landscape is the approval of lenacapavir (Yeztugo) as a twice‐yearly injectable pre‐exposure prophylaxis (PrEP) to prevent sexually acquired HIV‐1 in adults and adolescents at risk. This is a prevention (PrEP) indication, not treatment of people already living with HIV‐1.
Report Objectives
To visualize the global human immunodeficiency virus type-1 (HIV-1) treatment market segmentation based on type, treatment type, route of administration, distribution channel, and region as well as understand key commercial assets and players.
Identify commercial opportunities by analyzing trends and co-development
Excel data sheet with numerous data points of human immunodeficiency virus type-1 (HIV-1) treatment market-level with all segments.
PDF report consists of a comprehensive analysis after exhaustive qualitative interviews and an in-depth study.
Product mapping available as Excel consisting of key products of all the major players.
The global human immunodeficiency virus type-1 (HIV-1) treatment market report would provide approximately 70 tables,67 figures, and 187 Pages.
"Secure your 30% year-end discount - get this report before the offer expires."
:https://www.datamintelligence.com/buy-now-page?report=human-immunodeficiency-virus-type-1-treatment-market?pratik ((Purchase 2 or more Repots and get 50% Discount)
Market Segment Analysis
→ By Type (Group M, N, O, P)
In practice, nearly all commercial HIV‐1 treatment demand is associated with Group M viruses, with Groups N, O, and P remaining vanishingly rare in clinical practice.
Group M: Group M-associated infections account for essentially the whole treated HIV‐1 population; assigning about 99.8% of the 2025 market, this corresponds to roughly USD 38.4 billion.
Group N: Group N is extremely rare; for modeling, an assumption around 0.05% share (about USD 19 million) captures its negligible but non‐zero treated volume.
Group O: Group O is more prevalent than N in a few West‐Central African pockets but still very rare globally; allocating about 0.1% share (around USD 38 million) is reasonable.
Group P: Group P cases are extraordinarily rare; a working assumption of 0.05% (about USD 19 million) reflects effectively negligible commercial weight while keeping your type segmentation exhaustive.
→ By Treatment Type
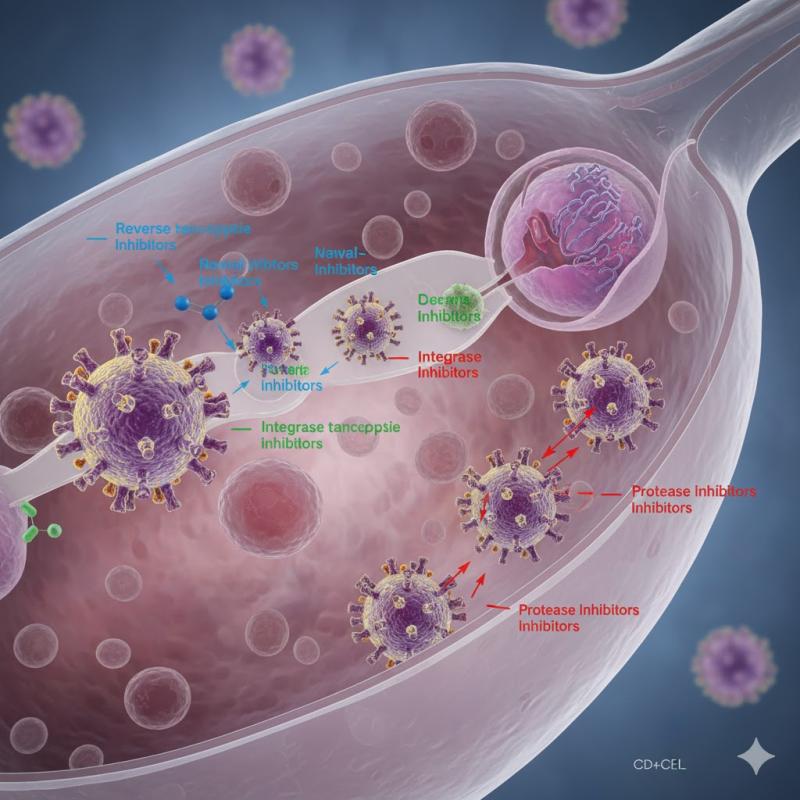
Published HIV therapeutics segmentation shows nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) as the largest single drug class, with substantial roles for NNRTIs, PIs, and INSTIs; combination regimens (fixed‐dose combinations and multi‐class ART) dominate actual prescribing. Mapping that to your structure and normalizing to 100% for 2025:
Combination therapies: Fixed‐dose and regimen‐level combinations are the backbone of modern ART and command the largest revenue pool when treated as a distinct "treatment type"; a pragmatic assumption is about 45% share, or USD 17.3 billion.
Protease inhibitors: PI‐based regimens are now more niche but remain important in certain patient segments; a share around 12% (≈USD 4.6 billion) aligns with their declining but still material contribution.
Integrase strand transfer inhibitors (INSTIs): INSTIs are the fastest‐growing class and widely used in first‐line and switch regimens; a working share of about 20% (USD 7.7 billion) reflects this strong uptake.
Nucleoside reverse transcriptase inhibitors (NRTIs): NRTIs underpin most ART backbones and hold the single largest class share in many reports; assigning 18% (USD 6.9 billion) to NRTI‐specific revenues fits with reported dominance once combination‐therapy revenues are separated out.
Non‐nucleoside reverse transcriptase inhibitors (NNRTIs): NNRTIs have lost share to INSTIs but remain widely used, particularly in resource‐limited settings; a modeled share of about 5% (USD 1.9 billion) is consistent with their shrinking yet persistent role.
Regional insights:-
• North America: 46% share . Driving factors include advanced healthcare infrastructure, widespread access to antiretroviral therapy (ART), high levels of awareness, and strong R&D plus government initiatives.
• Europe: 24% share . Growth supported by strong public health systems, early diagnosis/treatment initiatives, and ready access to modern HIV therapies.
• Asia-Pacific: 20% share . Growth driven by increasing HIV burden in populous countries, rising healthcare investment, improving access to ART, and growing treatment uptake.
Get Customization in the report as per your requirements:https://www.datamintelligence.com/customize/human-immunodeficiency-virus-type-1-treatment-market?pratik
Market Major Players
→ The major players in the human immunodeficiency virus type-1 (HIV-1) treatment market include Gilead Sciences, Inc., ViiV Healthcare, Janssen Global Services, LLC, Viatris Inc., Merck & Co., Inc., GSK plc., Bristol-Myers Squibb Company, Boehringer Ingelheim International GmbH, AbbVie. and CELLTRION INC. among others.
Key Developments:
→ In January 2025, the HIV-1 treatment market saw advancements in integrase strand transfer inhibitors (INSTIs) as preferred first-line therapies, with fixed-dose combinations gaining traction for improved patient adherence and reduced resistance risks. Market projections indicated growth from USD 39.1 billion in 2025, driven by simplified dosing regimens and public health initiatives expanding access in developing regions.
→ In September 2025, AI integration accelerated HIV-1 therapy development through machine learning for personalized treatments and drug discovery, with the broader HIV therapy market valued at around USD 36.4 billion amid rising adoption of automation in clinical trials. Future Market Insights highlighted sustained demand for novel antiretroviral combinations amid global efforts to curb transmission.
→ In November 2025, the Clinton Health Access Initiative's HIV Market Report revealed crisis-level disruptions in pediatric HIV services across 14 countries, with 26,000 children losing treatment in six months due to funding cuts, impacting overall HIV-1 treatment dynamics. This underscored challenges in maintaining market stability for vulnerable populations.
Request for 2 Days FREE Trial Access:
https://www.datamintelligence.com/reports-subscription?pratik
✅ Competitive Landscape
✅ Technology Roadmap Analysis
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Consumer Behavior & Demand Analysis
✅ Import-Export Data Monitoring
✅ Live Market & Pricing Trends
Have a look at our Subscription Dashboard:
https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About DataM Intelligence
DataM Intelligence is a renowned provider of market research, delivering deep insightsthrough pricing analysis, market share breakdowns, and competitive intelligence. Thecompany specializes in strategic reports that guide businesses in high-growth sectors suchas nutraceuticals and AI-driven health innovations.
To find out more, visit https://www.datamintelligence.com/ or follow us on Twitter,LinkedIn and Facebook.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Human Immunodeficiency Virus Type-1 (HIV-1) Treatment Market to reach US$ 62,752.56 million by 2031, North America led 46% of global market share | key Players:- Gilead Sciences, Inc., ViiV Healthcare, Janssen Global Services, LLC. here
News-ID: 4309492 • Views: …
More Releases from DataM intelligence 4 Market Research LLP

U.S. Alzheimer Drugs Market Set for Explosive Growth to USD 8.84 Billion by 2031 …
Leander Texas -
The Alzheimer Drugs Market reached US$ 4.46 billion in 2023 and is expected to reach US$ 18.33 billion by 2031, growing at a CAGR of 19.4% during the forecast period 2024-2031.
The Alzheimer's drugs market growth is driven by key US-Japan collaborations and approvals, including the JCR Pharma-Acumen Pharmaceuticals partnership to develop a novel blood-brain barrier Alzheimer therapy and expanded use of Eisai/Biogen's lecanemab with new subcutaneous application and…

Micro Nuclear Reactors (MNRs) Market to Reach US$ 4,865.85 Million by 2032 at 18 …
The Micro Nuclear Reactors (MNRs) Market reached US$ 1,434.55 million in 2024 and is projected to reach US$ 4,865.85 million by 2032, growing at a CAGR of 18.26 percent during the forecast period 2025 to 2032.
Market growth is driven by increasing demand for reliable, low-carbon energy solutions, supportive government policies promoting clean power generation, and the need for decentralized power in remote and industrial applications. Micro nuclear reactors offer scalable,…
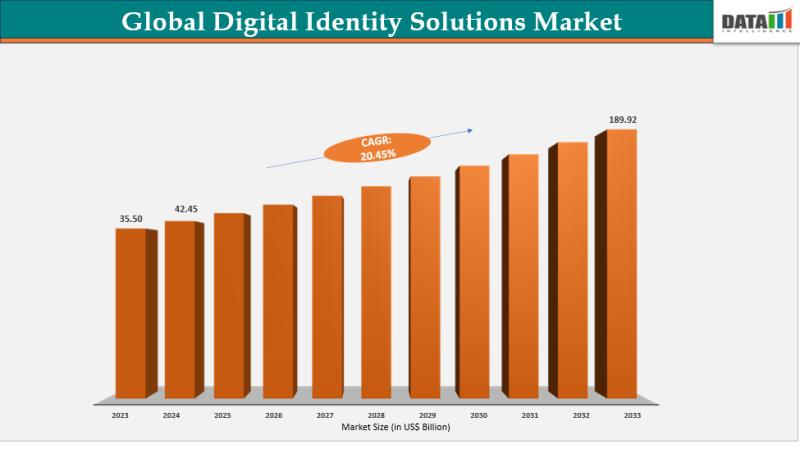
Digital Identity Solutions Market Set for Explosive Growth to US$189.92 Billion …
The Global Digital Identity Solutions Market reached US$35.50 billion in 2023, with a rise to US$42.45 billion in 2024, and is expected to reach US$189.92 billion by 2033, growing at a CAGR of 20.45% during the forecast period 2025-2033.
Market growth is driven by escalating cybersecurity threats, surging demand for secure authentication in remote work and e-commerce, and widespread adoption of biometric and blockchain-based verification. Advancements in AI-powered fraud detection, expanding…

Iron Ore Mining Market Set for Strong Growth to USD 620.7 Billion by 2031, Led b …
Leander Texas -
Iron Ore Mining Market reached US$ 330.2 billion in 2022 and is expected to reach US$ 620.7 billion by 2031, growing with a CAGR of 8.2% during the forecast period 2024-2031.
The Iron Ore Mining Market's strong growth is boosted by rising infrastructure and steel demand in the U.S. and Japan, coupled with strategic developments like Japanese firms acquiring stakes in global iron ore assets and collaborations with U.S.…
More Releases for HIV
HIV Drugs Market - Defeating HIV Together: Advancing Treatment Options for a Bri …
Newark, New Castle, USA: The "HIV Drugs Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
HIV Drugs Market: https://www.growthplusreports.com/report/hiv-drugs-market/7792
This latest report researches the industry structure, sales, revenue,…
HIV-Associated Lipodystrophy Treatment Market - Increasing prevalence of HIV is …
HIV-associated lipodystrophy also known as lipodystrophy is a syndrome that occurs in HIV-infected patients. It is characterized by loss of subcutaneous fat from face, buttocks, arms and legs. Although the exact cause of HIV-associated lipodystrophy is not fully elucidated, some research evidence reported that it occurs in HIV-infected patients who are under antiretroviral medications. According to an article published in National Center for Biotechnology Information (NCBI) in 2014, prevalence of…
Global HIV Drugs Market | Global HIV Drugs Industry | Global HIV Drugs Market Re …
Human immunodeficiency Virus (HIV) could be a chronic and severe sickness which might be transferred from one person to a different through blood-to-blood and sexual contact. it's a deadly disease that attacks immune cells called CD-4 cells, creating body vulnerable to infections and alternative diseases. Over the years, the rising prevalence of HIV sickness worldwide has completely influenced the demand for HIV medicine. HIV medicine facilitate in preventing the multiplication…
HIV Therapeutics Market– South Africa's Aspen launches three-in-one HIV drug
Recent Developments
Aspen Pharma care, a South Africa’s drug maker has launched a triple combination of tablet for the treatment of HIV in the country where the HIV virus is the most prevalent. The company's new Emdolten drug is a once a day tablet which is in the form of dolutegravir, an antiretroviral medication that balances the drug’s resistance. The company has launched Aspen Stavudine which was its first generic ARV…
HIV Vaccine Market HIV Vaccine Clinical Pipeline Report 2022
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
Introduction to Human Immunodeficiency Virus (HIV) Vaccines
1.1 Overview
1.2 Antiquity of HIV Vaccine
Need for the Development of HIV Vaccine
Primer of HIV inside the Body
3.1 Inclusion of HIV Virus into the System
3.2 Interaction of HIV with Host
3.3 Eradication of HIV Virus
HIV Vaccine Development Process
4.1 Introduction
…
Global HIV Vaccine Market & HIV Vaccine Clinical Trial Outlook 2022
Worldwide, around the 35 Million of the people are currently infected with the HIV and about 30 Million of the people died because of the AIDS infection. There is no human example of clearing an HIV infection naturally. HIV virus makes copies of it very quickly, many types of HIV exist and new types of virus are continue to rise. Many scientists are still trying to understand the specific ways…