Press release
European In Vitro Diagnostic Packaging Market Outlook 2025-2035: Key Developments and Future Scope
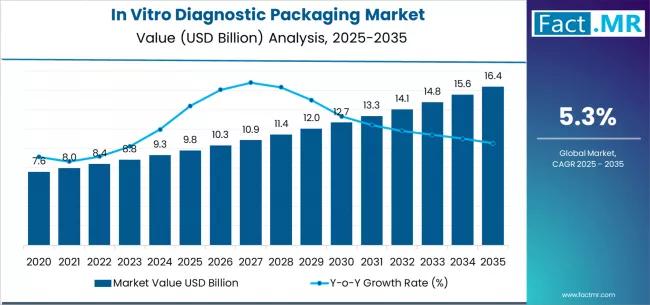
The in vitro diagnostic (IVD) packaging market is poised for steady expansion through 2035 as global demand for diagnostic testing, point-of-care (POC) solutions, and high-throughput laboratory workflows continues to grow. IVD packaging plays a critical role across the diagnostics value chain - protecting reagents and disposables, preserving sample integrity, ensuring sterility, enabling accurate assay performance, and meeting regulatory and cold-chain requirements. From microtiter plates, cartridges, and reagent vials to trays, blister packs, and diagnostic kit boxes, packaging choices influence shelf life, transportability, clinical usability, and end-user confidence.The market is benefiting from several converging trends: decentralization of testing into clinics, pharmacies, and homes; growth of rapid antigen and molecular POC tests; proliferation of self-collection kits; and increasing regulatory scrutiny on labeling, traceability, and temperature-controlled logistics. Suppliers that can deliver validated, contamination-free, traceable, and sustainable packaging solutions are expected to capture a growing share of IVD demand.
To Access the Complete Data Tables & in-depth Insights, Request a Discount on this report: https://www.factmr.com/connectus/sample?flag=S&rep_id=12339
Quick Stats (2025-2035):
Market Value 2025: Significant multi-billion-dollar value driven by reagent and kit packaging demand.
Forecast Growth Through 2035: Strong expansion reflecting rising POC testing, home diagnostics, and centralized lab throughput.
Forecast CAGR (2025-2035): Mid-single digits to low-double digits depending on region and product mix.
Leading Packaging Types: Cartridge & cassette packaging, vial & ampoule systems, and blister/foil pouches for single-use reagents.
Key Growth Drivers: POC testing proliferation, self-collection kits, cold-chain logistics, regulatory traceability, and sustainability demands.
Market Drivers
1. Shift Toward Decentralized & Point-of-Care Testing
Rapid and molecular POC platforms, lateral flow tests, and cartridge-based molecular assays are expanding into clinics, pharmacies, and home settings. These formats require compact, tamper-evident, and user-friendly packaging-often with integrated instructions, single-use dispensing features, and clear waste-management labeling.
2. Surge in Self-Collection & At-Home Diagnostics
Self-collection kits for respiratory pathogens, STIs, and chronic-disease monitoring (e.g., HbA1c, fertility testing) demand secure sample collection devices, biohazard-safe envelopes, and robust return-shipping packaging. Ensuring sample integrity during transit is crucial, driving innovation in cushioning, desiccants, and temperature-stable materials.
3. Cold-Chain & Temperature Control Needs
Many IVD reagents and enzyme-based assays require controlled temperatures. Growth in biologic reagents and temperature-sensitive molecular kits increases demand for insulated shippers, phase-change materials, active cooling systems, and validated cold-chain packaging solutions that meet regulatory documentation standards.
4. Regulatory Traceability & Labeling Requirements
Stringent requirements for lot traceability, expiration visibility, human-readable instructions, barcoding, and tamper evidence place a premium on packaging that supports serialization, RFID tagging, QR codes, and secure labeling-especially for high-value diagnostic consumables.
5. Sustainability & Waste Management Pressure
Healthcare purchasers and regulators increasingly demand recyclable materials, reduced plastic footprints, and designs that minimize hazardous-waste volume. Packaging suppliers are innovating with mono-material formats, lightweight insulation, and recyclable cushioning to balance performance with environmental goals.
Market Structure & Segment Insights:
By Packaging Type
Cartridges & Cassettes: Increasingly used in rapid molecular and POC instruments; require precision molding and sterile inner liners.
Vials, Ampoules & Reagent Bottles: Core to centralized labs and high-throughput platforms; demand for leak-proof closures and pharmaceutical-grade materials remains high.
Blister & Foil Pouches: Common for single-use reagents and lateral-flow strips; heat-sealable, moisture-barrier pouches are standard.
Cold-Chain Solutions: Insulated boxes, gel packs, and active cooling units are growing with the rise of enzyme and reagent shipments.
By End-User
Hospitals and centralized laboratories continue to drive high-volume packaging demand, while POC settings, retail test providers, and direct-to-consumer channels accelerate adoption of compact, consumer-friendly packaging formats.
By Region:
North America and Europe lead in value due to early adoption of advanced diagnostics and strict regulatory requirements. Asia-Pacific is the fastest-growing region driven by expanding laboratory networks, growing POC infrastructure, and rising domestic manufacturing of tests and kits.
Challenges & Constraints:
Compatibility & Validation Burden
IVD packaging must be validated with specific assays to ensure no interaction with reagents, no adsorption of analytes, and preserved assay performance-creating technical hurdles and longer development cycles for new packaging formats.
Regulatory Documentation & Compliance Costs
Serialization, stability validation, and cold-chain qualification require investment in testing, documentation, and quality management - costs that can be substantial for smaller packaging firms.
Medical Waste & Disposal Regulations
Single-use packaging contributes to clinical waste streams. Designing packaging that balances sterility and disposability with recycling or reduced waste complexity is a difficult engineering and regulatory challenge.
Opportunities & Strategic Directions:
Integrated Smart Packaging
Embedding sensors (temperature loggers), QR/ RFID traceability, and user-guidance via smartphone scanning improves chain-of-custody, patient instructions, and post-market surveillance-valuable to manufacturers and healthcare systems.
Mono-Material & Recyclable Solutions
Developing mono-material pouches and recyclable tray systems that meet sterility and barrier needs will create competitive advantage as sustainability becomes procurement criteria.
Custom Kitting & Turnkey Solutions
Offering end-to-end services-kit assembly, labeling, serialization, cold-chain fulfillment, and clinician/user-centric instructions-helps packaging suppliers lock in long-term partnerships with diagnostic OEMs.
Localized Cold-Chain & On-Demand Manufacturing
Regionalized packaging and fulfillment hubs reduce transit time for temperature-sensitive reagents and support rapid scale-up during public-health responses or product launches.
Outlook:
The IVD packaging market is set to grow in tandem with broader diagnostics expansion-particularly as testing decentralizes, self-collection and home testing rise, and temperature-sensitive molecular assays proliferate. Suppliers that combine validated material science, robust cold-chain solutions, traceability features, and sustainable design will capture the largest share of this evolving market. As healthcare systems demand faster, safer, and more environmentally responsible diagnostic kits, packaging will remain a strategic differentiator for IVD manufacturers and their supply-chain partners.
Browse Full Report: https://www.factmr.com/report/in-vitro-diagnostic-packaging-market
Purchase Full Report for Detailed Insights
For access to full forecasts, regional break-outs, product- and application-level analysis, company share details, and emerging trend assessments, you can purchase the complete report: https://www.factmr.com/checkout/12339
Have specific requirements or need assistance on report pricing or have a limited budget? Please contact sales@factmr.com
Related Reports:
Infection Control Market: https://www.factmr.com/report/infection-control-market
Intravenous Infusion Pumps Market: https://www.factmr.com/report/intravenous-infusion-pumps-market
Intravenous Iron Drugs Market: https://www.factmr.com/report/intravenous-iron-drugs-market
Inflammatory Bowel Disease (IBD) Treatment Market: https://www.factmr.com/report/inflammatory-bowel-disease-ibd-treatment-market
Contact:
US Sales Office
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583, +353-1-4434-232
Email: sales@factmr.com
About Fact.MR:
Fact.MR is a global market research and consulting firm, trusted by Fortune 500 companies and emerging businesses for reliable insights and strategic intelligence. With a presence across the U.S., UK, India, and Dubai, we deliver data-driven research and tailored consulting solutions across 30+ industries and 1,000+ markets. Backed by deep expertise and advanced analytics, Fact.MR helps organizations uncover opportunities, reduce risks, and make informed decisions for sustainable growth.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release European In Vitro Diagnostic Packaging Market Outlook 2025-2035: Key Developments and Future Scope here
News-ID: 4304800 • Views: …
More Releases from Fact.MR
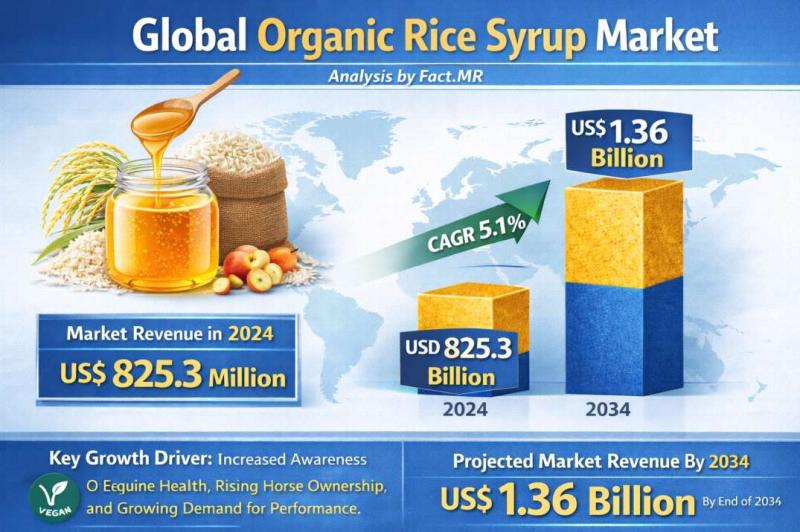
Organic Rice Syrup Market is forecasted to increase at a CAGR of 5.1% and US$ 1. …
The global Organic Rice Syrup Market is projected to expand steadily over the coming decade, driven by rising consumer demand for natural, clean-label sweeteners and growing awareness of health and wellness trends. Industry analysts estimate that the organic rice syrup market, valued at approximately USD 450 million in 2025, is expected to reach nearly USD 880 million by 2035, registering a compound annual growth rate (CAGR) of about 7.1% during…
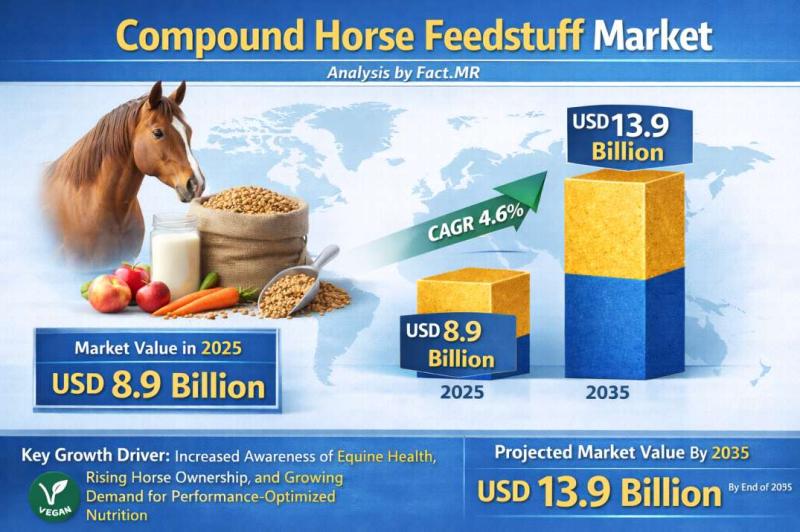
Compound Horse Feedstuff Market is Estimated to Grow at a CAGR of 4.6%, Reaching …
The global compound horse feedstuff market is galloping toward steady growth, projected to expand from a valuation of USD 3.8 billion in 2026 to approximately USD 5.4 billion by 2036. This represents a compound annual growth rate (CAGR) of 3.6% over the ten-year forecast period.
The market is being driven by the "humanization" of equine companions, the professionalization of equestrian sports, and a significant shift toward specialized performance nutrition that…
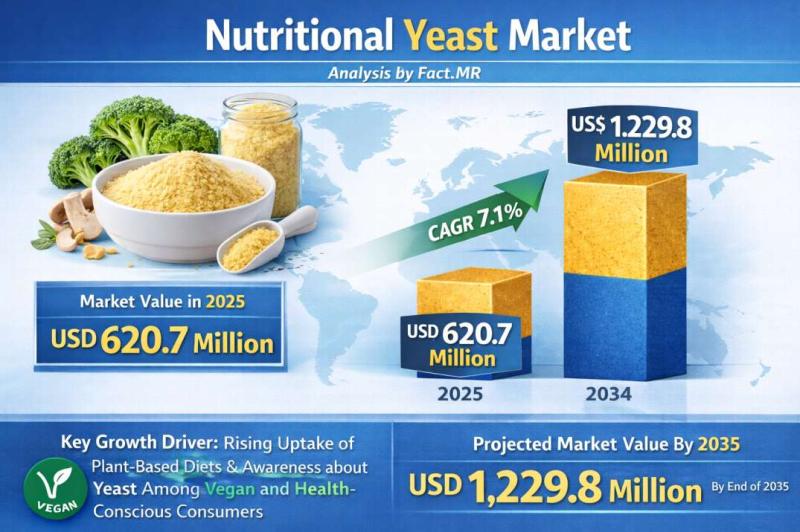
Nutritional Yeast Market Forecasted CAGR is 7.1% by 2035 | Fact.MR Report
The global nutritional yeast market is experiencing a significant surge in demand, projected to grow from a valuation of USD 515.2 million in 2026 to approximately USD 1.2 billion by 2036. This represents a robust compound annual growth rate (CAGR) of 8.8% over the ten-year forecast period.
The market is being propelled by the global explosion of plant-based diets and the "clean-label" movement, with nutritional yeast emerging as the primary…
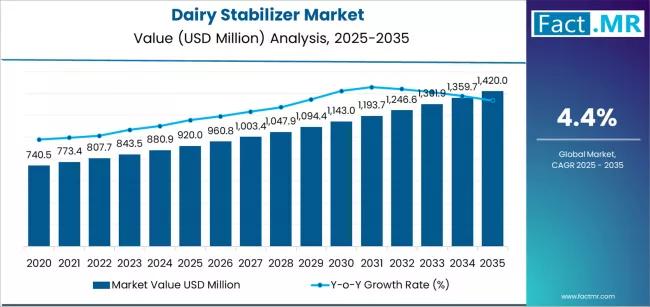
Dairy Stabilizer Market is Expected to Reach USD 1,420.0 million by 2035 | Resea …
The global Dairy Stabilizers Market is projected to sustain solid growth over the next decade as consumer demand for high-quality dairy and dairy-based products continues to expand across foodservice and retail sectors. Industry analysts estimate that the dairy stabilizers market, valued at approximately USD 2.4 billion in 2025, is expected to reach around USD 4.3 billion by 2035, registering a compound annual growth rate (CAGR) of about 6.5% during the…
More Releases for POC
Primary Catalyst Driving POC Medical Imaging Market Evolution in 2025: Chronic D …
What market dynamics are playing a key role in accelerating the growth of the poc medical imaging market?
The escalating occurrence of long-term diseases is predicted to boost the expansion of the point-of-care medical imaging market in the future. The chronic disease prevalence pertains to the fraction or the count of people in a community affected by enduring health ailments at any given moment. This transition originates from lifestyle options, inherited…
Transforming the Point-of-Care (POC) Coagulation Testing Market in 2025: Cardiov …
"What Is the Expected Size and Growth Rate of the Point-of-Care (POC) Coagulation Testing Market?
The point-of-care (POC) coagulation testing market has grown significantly in recent years. It will grow from $1.18 billion in 2024 to $1.25 billion in 2025, at a CAGR of 5.8%. The growth is driven by increasing patient demand, the growing trend of point-of-care testing, high reliability in coagulation status testing, automation, integration with electronic health records…
POC Diagnostic Testing Devices | 1drop
1drop Inc.'s POC diagnostic testing devices are essential tools in modern healthcare, designed to deliver quick and accurate results for various health conditions at the point of care. These devices make diagnostics more accessible and timelier, enhancing healthcare delivery and patient outcomes.
Point-of-Care Molecular Diagnostic Platform
1drop offers a comprehensive POC molecular diagnostic platform that consists of three main components:
• Reagent: Specialized reagents formulated for specific tests, including molecular diagnostics.
• Device: The 1POT™ Duo…
POC Diagnostics Market Size 2024 to 2031.
Market Overview and Report Coverage
POC, or Point of Care, Diagnostics refer to medical tests that are performed outside of a traditional laboratory setting, allowing for quicker results and immediate treatment decisions. The POC Diagnostics Market encompasses a wide range of diagnostic tests including blood glucose monitoring, pregnancy tests, infectious disease testing, and more.
The future outlook of the POC Diagnostics Market is optimistic, with the market expected to grow…
Point of Care (POC) Coagulation Testing Devices Market - Transforming Coagulatio …
Newark, New Castle, USA: The "Point of Care (POC) Coagulation Testing Devices Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Point of Care (POC) Coagulation Testing Devices…
POC Cardiovascular Diagnostic Market 2022 | Detailed Report
According to Market Study Report, POC Cardiovascular Diagnostic Market provides a comprehensive analysis of the POC Cardiovascular Diagnostic Market segments, including their dynamics, size, growth, regulatory requirements, competitive landscape, and emerging opportunities of global industry. An exclusive data offered in this report is collected by research and industry experts team.
Download FREE Sample Report @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=5467652
The report provides a comprehensive analysis of company profiles listed below:
- Abbott
- Biomerieux
- Danaher
- F.…