Press release
European RNA Therapy Clinical Trials Market Outlook 2025-2035: Innovation, Growth, and Demand Trends
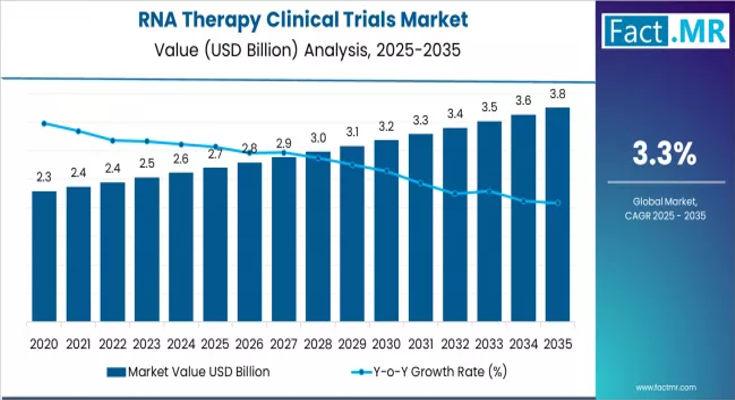
The global RNA therapy clinical trials market is projected to expand significantly over the next decade, driven by rising interest in genetic medicine, increasing investment in RNA-based platforms, and growing demand for personalized therapies. From an estimated USD 2.70 billion in 2025, the market is forecast to reach approximately USD 3.75 billion by 2035, reflecting a compound annual growth rate (CAGR) of about 3.3%.Key Market Highlights
2025 Market Size: USD 2.70 billion
2035 Forecast Value: USD 3.75 billion
Projected CAGR (2025-2035): ~3.3%
Leading Modality: Messenger RNA (mRNA) (~34.8% share)
Top Therapeutic Focus: Rare diseases
High-Growth Regions: Asia Pacific, Europe, North America
Major Service Providers: IQVIA; ICON Plc; Laboratory Corporation of America; Charles River Laboratories; PAREXEL; Syneos Health; Medpace; PPD; Novotech; Veristat
To Access the Complete Data Tables & in-depth Insights, Request a Discount on this report: https://www.factmr.com/connectus/sample?flag=S&rep_id=11697
Major Growth Drivers:
Surging Adoption of RNA-Based Therapeutics
Biotech and pharmaceutical companies are increasingly focusing on RNA platforms-such as mRNA, siRNA, and antisense molecules-because of their ability to target previously "undruggable" genes, enable rapid development, and customize therapies for individual patients. These platforms are becoming central to next-generation drug development.
Expansion of Clinical Capabilities for Rare Diseases
Rare and genetic disorders are driving the demand for RNA therapy trials. Given the specificity of RNA-based agents, they are well-suited for treating rare conditions, prompting sponsors to design specialized trials for small patient populations and novel indications.
Increasing Investment in Precision Medicine
Pharmaceutical sponsors are investing more in precision medicine. RNA therapies offer a highly modular and flexible approach to treatment, and companies are seeking clinical trial partners with expertise in RNA delivery, regulatory strategy, and patient recruitment.
Regulatory Momentum & Delivery Innovation
Regulatory agencies are adapting to the rise of RNA therapies, and delivery technologies such as lipid nanoparticles, polymeric carriers, and advanced conjugates are reducing barriers to clinical translation. These improvements are making RNA trials safer and more effective.
Growing CRO Specialization
Contract research organizations (CROs) have begun to specialize in RNA-focused clinical services. Their expertise in trial design, manufacturing support, and regulatory navigation is becoming increasingly critical for sponsors developing RNA therapeutics.
Market Segmentation
By Modality
Messenger RNA (mRNA): Dominant modality due to applications in gene expression, vaccines, and therapeutics.
siRNA / RNA Interference: Widely used for disease suppression and gene silencing.
Antisense Oligonucleotides (ASOs): Gaining traction for precision and targeted gene modulation.
Other RNA Types: Include microRNA-based therapies and RNA editing modalities.
By Therapeutic Area
Rare Diseases: The largest area, driven by genetic disorder treatments.
Oncology: RNA therapies targeting cancer are rising rapidly.
Infectious Diseases: Vaccines and antiviral RNA drugs remain key targets.
Neurological Diseases: Growing pipeline for neurodegenerative and rare neurological conditions.
By Trial Phase
Phase I: Early safety and dose-finding trials.
Phase II (largest share): Demonstrating efficacy and optimizing dose in selected patient populations.
Phase III: Large-scale trials aimed at regulatory approval.
Phase IV / Post-Marketing: Long-term safety and real-world data collection.
By Region
North America: Leading region, supported by mature biotech infrastructure and significant R&D investment.
Europe: Strong trial activity, especially in rare diseases and oncology, backed by regulatory harmonization.
Asia Pacific: Rapid growth, driven by increasing biotech capacity, lower trial costs, and expanding regulatory experience.
Challenges & Risks
High Development Complexity: RNA therapies require specialized delivery systems and manufacturing processes, increasing cost and time.
Regulatory Uncertainty: As novel modalities, RNA therapies still face evolving regulations, especially for first-in-class drugs.
Patient Recruitment Issues: Recruiting patients for rare diseases can be challenging and slow for clinical trials.
Safety & Immunogenicity: RNA drugs may trigger immune responses or off-target effects, making trial design more complex.
Competitive Landscape
Key players in the RNA clinical trial services market are focusing on:
Expanding capabilities for RNA delivery, manufacturing, and clinical execution
Forming strategic partnerships with biotech firms developing RNA therapeutic candidates
Developing fully integrated platforms to support phase I through phase III development
Investing in regulatory expertise and data infrastructure to support complex RNA trials
Building global trial networks, especially in regions with favorable regulatory and cost environments
Strategic Recommendations
Develop RNA-Optimized Trial Platforms: CROs should invest in RNA-specific infrastructure, from LNP management to mRNA-specific analytical methods.
Build Rare Disease Capabilities: Develop deep expertise in designing and enrolling patients for rare-disease RNA trials.
Form Long-Term Partnerships: Collaborate with biotech firms early to co-develop novel RNA therapies and share regulatory knowledge.
Enhance Regulatory Strategy: Invest in regulatory teams that specialize in RNA modalities to help sponsors navigate complex approval pathways.
Expand Regionally: Focus on establishing trial operations in Asia Pacific and Europe to tap into growing RNA therapy development globally.
Market Outlook
By 2035, the RNA therapy clinical trials market is expected to reach USD 3.75 billion, marking a significant step forward in the evolution of genetic medicine. Driven by mRNA innovation, rare-disease targeting, and advanced clinical capabilities, RNA therapies are becoming central to modern drug development.
Contract research organizations, biotech companies, and pharmaceutical sponsors that align their strategy with RNA specializations will be best positioned to capitalize on this transformative opportunity-delivering precision therapeutics that address unmet medical needs and shape the future of medicine.
Browse Full Report: https://www.factmr.com/report/rna-therapy-clinical-trials-market
Purchase Full Report for Detailed Insights
For access to full forecasts, regional break-outs, product- and application-level analysis, company share details, and emerging trend assessments, you can purchase the complete report: https://www.factmr.com/checkout/11697
Have specific requirements or need assistance on report pricing or have a limited budget? Please contact sales@factmr.com
Related Reports:
RNA Editing for Neurodegenerative Diseases Market: https://www.factmr.com/report/rna-editing-for-neurodegenerative-diseases-market
RNA Editing Therapies Market: https://www.factmr.com/report/rna-editing-therapies-market
RNA Transcriptome Profiling Test Market: https://www.factmr.com/report/3113/rna-transcriptome-profiling-test-market
RNA-based Therapeutics and Vaccine Market: https://www.factmr.com/report/rna-based-therapeutics-and-vaccine-market
Contact:
US Sales Office
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583, +353-1-4434-232
Email: sales@factmr.com
About Fact.MR:
Fact.MR is a global market research and consulting firm, trusted by Fortune 500 companies and emerging businesses for reliable insights and strategic intelligence. With a presence across the U.S., UK, India, and Dubai, we deliver data-driven research and tailored consulting solutions across 30+ industries and 1,000+ markets. Backed by deep expertise and advanced analytics, Fact.MR helps organizations uncover opportunities, reduce risks, and make informed decisions for sustainable growth.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release European RNA Therapy Clinical Trials Market Outlook 2025-2035: Innovation, Growth, and Demand Trends here
News-ID: 4280845 • Views: …
More Releases from Fact.MR
Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
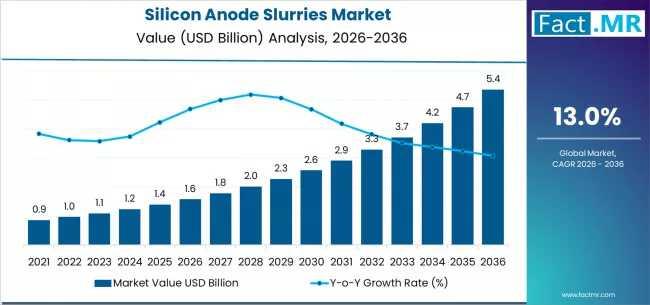
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…

Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…
Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
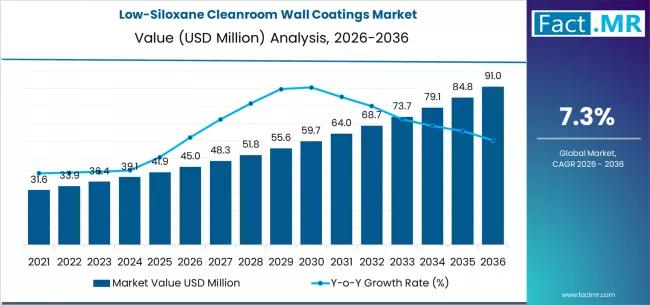
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…

Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…
More Releases for RNA
CD Formulation Launches Custom Circular RNA Synthesis Service to Accelerate RNA …
CD Formulation introduces a customizable circRNA synthesis service, delivering high-quality, stable circRNAs for therapeutics, vaccines, and gene research, supported by advanced design and QC processes.
CD Formulation, a leading provider of advanced small nucleic acid synthesis [https://www.formulationbio.com/nucleic-acid/custom-small-nucleic-acid-synthesis.html] solutions, is proud to announce the launch of its fully customizable circular RNA (circRNA) synthesis service. This new service addresses the growing need for stable, non-immunogenic RNA molecules for therapeutic development, vaccine research, and…
Self-Amplifying RNA Synthesis Market Gains Traction as Biotech Firms Embrace Sca …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " Self-Amplifying RNA Synthesis Market- (By Product & Service (Products (Enzymes & Reagents, Premade saRNA, Others), Custom Synthesis Services), By Application (Therapeutics Development (Oncology, Infectious Diseases, Others), Biomedical Research), By End-User (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic,…
RNA Extraction and RNA Purification Market: Growth, Trends & Competitive Landsca …
The global RNA Extraction and RNA Purification Market is expected to grow at 6.3% CAGR from 2025 to 2032.
This Market Report is the result of extensive research and analysis conducted by our team of experienced market researchers through -
• 70% efforts of Primary Research
• 15% efforts of Secondary Research
• 15% efforts from the subscription to Paid database providing industry overview, macro and micro economics factors, and financials of private limited…
RNA Targeting Small Molecules Therapeutics Market: Exponential Growth with Risin …
Estimations Predict a CAGR of 29.8% by 2029 in Global RNA Targeting Small Molecules Therapeutics Market Boosted by Precision Medicine, RNA Biomarker Identification and RNA Genetic Manipulation
What Is The Projected Market Size of The Global RNA Targeting Small Molecules Therapeutics Market And Its Growth Rate?
• The market will grow from $6.1 billion in 2024 to $7.87 billion in 2025 at a compound annual growth rate (CAGR) of 28.9%.
• Expected exponential…
Global DNARNA Extraction Kit Market by Type (Cell-free DNA (cfDNA), Sequence-spe …
"DNARNA Extraction Kit Market" is segmented by Company, Region (country), By Type, Application, stakeholders and other participants. This report provides an analysis of revenue and forecast across Type and Application segments for 2023-2032.
The market for DNARNA Extraction Kits has been thoroughly researched via primary and secondary sources to produce this research study. Along with a competitive analysis of the market, segmented by application, type, and geographical trends, it offers a…
Cancer RNA Expression Market to Reap Excessive Revenues by 2028(By sequencing te …
Worldwide cancer is one of the leading cause of death and effective way of treating it still looks unaccomplished in most parts of the world. The factors which influence the successful treatment of cancer are different depending on the stage of diagnosis, treatment availability and availability of trained healthcare professionals coupled with high economic burden of the disease. The gene expression of cancerous cells varies by cancer type and may…