Press release
Nucleic Acid Therapeutics Market Set to Surge to USD 44.5 Billion by 2035 as Gene- and RNA-Based Therapies Move from Promise to Practice | TMR
The global Nucleic Acid Therapeutics Market is entering a phase of rapid expansion, propelled by scientific breakthroughs, growing incidence of genetic disorders, and a regulatory environment that increasingly supports expedited development pathways. Valued at US$ 8.8 billion in 2024, the market is forecast to grow at a CAGR of 14.7% between 2025 and 2035, reaching an estimated US$ 44.5 billion by 2035.Review significant findings and insights from our Report in this sample -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86333
Nucleic acid therapies - encompassing antisense oligonucleotides (ASOs), small interfering RNA (siRNA), gene therapies, aptamers, and other RNA/DNA-based modalities - are transforming the therapeutic landscape by directly targeting disease at the genetic or transcriptomic level. Their rise reflects a broader shift toward precision medicine and tailored interventions for previously intractable diseases, from rare inherited disorders to oncology and infectious disease.
Market Dynamics: Biology, Clinical Success, and Regulatory Momentum
Three converging forces underlie the market's strong outlook: the growing prevalence and diagnosis of genetic disorders, accelerating clinical successes across multiple modalities, and increasingly supportive regulatory frameworks that prioritize therapies for high unmet need.
Better diagnostic technologies and wider genetic screening are expanding the identifiable patient populations for monogenic and rare disorders. At the same time, high-profile clinical and commercial successes - notably in RNA therapeutics and gene replacement strategies - have demonstrated that nucleic acid-based approaches can deliver meaningful clinical benefit. This proof of concept is encouraging investment and partnership activity across large pharma, biotech, and smaller specialist developers.
Regulators including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have expanded tools such as Fast Track, Breakthrough Therapy, and Accelerated Approval pathways that reduce time to market for therapies addressing severe or rare diseases. These mechanisms, combined with adaptive trial designs and growing real-world evidence capabilities, are materially lowering barriers for innovative nucleic acid therapeutics.
Therapy-Type Leadership: ASOs Take Early Lead; siRNA and Gene Therapies Accelerate
Among therapy types, antisense oligonucleotides (ASOs) currently dominate market share due to established clinical programs and multiple approved products addressing rare genetic conditions. ASOs' ability to selectively modulate RNA function - by promoting degradation, altering splicing, or blocking translation - has produced validated therapies for several neuromuscular and metabolic disorders, underpinning ASOs' leading role.
However, siRNA and gene therapies are rapidly gaining momentum. Advances in chemical modification, lipid- and polymer-based delivery systems, and targeted conjugates are overcoming historical delivery and stability challenges for RNA interference. Gene therapies, powered by refined viral vectors (e.g., AAV) and genome-editing platforms, are expanding beyond ultra-rare diseases into larger patient populations where durable, potentially one-time treatments are scientifically feasible.
Uncover valuable insights by exploring our comprehensive report -
https://www.transparencymarketresearch.com/nucleic-acid-therapeutics-market.html
Emerging modalities - including aptamers and mRNA therapeutics for non-vaccine indications - are also maturing and will add to market breadth as manufacturing, cost, and regulatory pathways normalize.
Delivery Methods: Viral and Non-Viral Platforms Evolve in Tandem
Delivery remains a central technical challenge and competitive battleground. The market supports two principal delivery approaches: viral vector-based systems, which excel at durable gene delivery to select tissues, and non-viral systems, which include lipid nanoparticles (LNPs), conjugates (e.g., GalNAc), polymers, and physical methods.
Viral vectors, particularly adeno-associated virus (AAV) platforms, are the workhorses of in vivo gene replacement and long-duration expression strategies. Improving vector design, capsid engineering, and manufacturing scale are priorities that have reduced immunogenicity and broadened tissue tropism.
Non-viral delivery has seen rapid progress following the high-profile success of mRNA LNP vaccines. Chemical conjugation (such as GalNAc for hepatocyte targeting) and advanced nanoparticle formulations now enable safe, efficient systemic delivery of RNA therapeutics. These non-viral platforms are especially attractive for siRNA, ASOs, and mRNA therapeutics where repeated dosing or rapid manufacturing is advantageous.
Therapeutic Areas: From Rare Diseases to Oncology and Beyond
Nucleic acid therapeutics maintain especially strong traction in neuromuscular, metabolic, and ophthalmologic disorders, where single-gene defects are well-characterized and small patient populations can justify high-value therapies. Nevertheless, the technology is rapidly expanding into oncology, cardiovascular, infectious disease, and autoimmune indications.
Oncology offers particular scale potential through approaches such as RNA-based immunomodulation, targeted siRNA programs against oncogenic drivers, and combination regimens that pair nucleic acid drugs with checkpoint inhibitors or cell therapies. Infectious disease applications - building on mRNA vaccine platforms - show promise for rapid-response therapeutics and prophylactics.
Regional Outlook: North America Leads, Globalization Accelerates
North America currently commands the largest share of the nucleic acid therapeutics market, driven by a concentrated ecosystem of biotech hubs, deep venture capital and corporate R&D investment, and favorable regulatory engagement. The region's robust clinical trial infrastructure and high adoption rates for cutting-edge therapies underpin this leadership.
Nevertheless, growth is globalizing. Europe, Asia Pacific, Latin America, and select MENA markets are increasing clinical capacity, manufacturing capability, and regulatory maturity. Strategic collaborations, local manufacturing investments, and licensing deals are accelerating market access in China, Japan, India, and emerging markets, expanding patient reach and diversifying development pipelines.
Competitive Landscape: Big Pharma, Biotech, and New Entrants
The competitive landscape blends established pharmaceutical companies and specialized biotech pioneers. Notable players include Novartis, Pfizer, Sanofi, AstraZeneca, Alnylam Pharmaceuticals, Amgen, Sarepta Therapeutics, Bluebird Bio, and PTC Therapeutics, among others. These organizations are pursuing diverse strategies - in-house R&D, acquisitions, platform licensing, and co-development alliances - to secure leadership in specific modalities and indications.
Strategic activity is robust: partnerships that pair discovery platforms with advanced delivery technologies, M&A to capture vector or LNP capabilities, and licensing deals that broaden geographic access to promising clinical-stage assets.
Recent Developments Illustrating Market Momentum
Industry dealmaking and corporate advancements demonstrate the market's vibrancy. For example, recent high-value transactions and licensing agreements have fortified pipelines across large and mid-sized companies, expanding access to siRNA and gene therapy candidates and accelerating clinical development in neuromuscular and rare pulmonary diseases. (User-supplied, illustrative corporate actions in 2024 underscore the trend toward consolidation and strategic alliances.)
Challenges and Restraints: Cost, Manufacturing, and Access
Despite strong momentum, the nucleic acid therapeutics market faces key challenges. High manufacturing costs - especially for viral vectors and clinical-grade nucleic acids - remain a barrier to broad access. Long-term safety monitoring, immune responses to vectors, and off-target effects for gene-editing approaches require careful clinical design and regulatory oversight.
Payer frameworks are still evolving to manage one-time gene therapies and chronic dosing regimens for RNA drugs. Ensuring equitable global access will depend on reduced manufacturing costs, flexible pricing models, and outcomes-based reimbursement structures.
Opportunities: Platform Technology, Manufacturing Scale, and Combination Therapies
Opportunities abound for companies that can scale manufacturing, refine delivery systems, and demonstrate durable, clinically meaningful outcomes. Platform technologies that enable rapid candidate generation, improved tissue targeting, and lower-cost production will capture outsized market value. Combination strategies - pairing nucleic acid therapeutics with small molecules, biologics, or cell therapies - offer new therapeutic synergies and broadenable indications.
Further, expanding indications into larger patient populations (e.g., oncology, common metabolic disorders) will materially enlarge market size beyond rare-disease niches.
Buy this Premium Research Report to explore detailed market trends -
https://www.transparencymarketresearch.com/checkout.php?rep_id=86333<ype=S
Analyst Perspective
Analysts view the nucleic acid therapeutics market as a multi-decade opportunity. Continued scientific progress, improved delivery and manufacturing, and increasingly adaptive regulatory frameworks create a favorable environment for durable commercial success. Companies that invest in scalable platforms, secure efficient delivery franchises, and demonstrate reproducible clinical outcomes will be best positioned to capture the significant upside projected through 2035.
Explore Latest Research Reports by Transparency Market Research:
Animal Model Market - https://www.transparencymarketresearch.com/animal-model-market.html
Multi-omics Market - https://www.transparencymarketresearch.com/multiomics-market.html
Viral Vectors & Plasmid DNA Manufacturing Market - https://www.transparencymarketresearch.com/viral-vectors-plasmid-dna-manufacturing-market.html
Bioprocess Validation Market - https://www.transparencymarketresearch.com/bioprocess-validation-market.html
Forensic Genomics Market - https://www.transparencymarketresearch.com/forensic-genomics-market.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
Follow Us: LinkedIn| Twitter| Blog | YouTube
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Nucleic Acid Therapeutics Market Set to Surge to USD 44.5 Billion by 2035 as Gene- and RNA-Based Therapies Move from Promise to Practice | TMR here
News-ID: 4278271 • Views: …
More Releases from Transparency Market Research
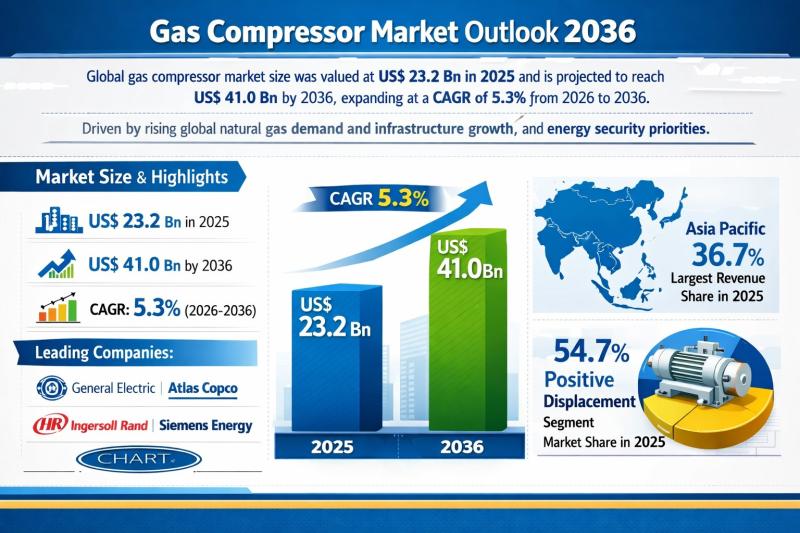
Gas Compressor Market Outlook 2036: Global Industry Expected to Reach US$ 41.0 B …
The global gas compressor market was valued at US$ 23.2 Bn in 2025 and is projected to reach US$ 41.0 Bn by 2036, expanding at a compound annual growth rate (CAGR) of 5.3% from 2026 to 2036. This steady growth trajectory reflects the structural importance of gas compression systems across upstream, midstream, and downstream gas value chains. Rising natural gas consumption, expansion of pipeline and LNG infrastructure, and national energy…
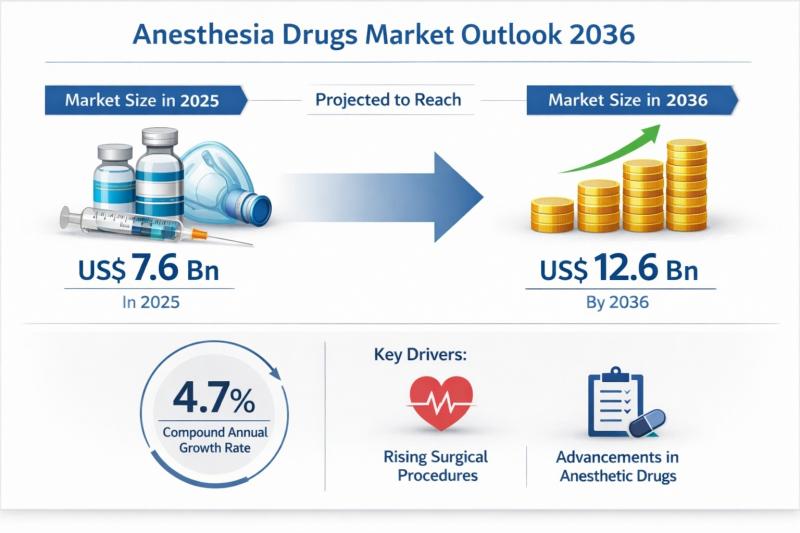
Anesthesia Drugs Market to be Worth USD 12.6 Bn by 2036 - By Drug / By Applicati …
The global anesthesia drugs market was valued at US$ 7.6 billion in 2025 and is projected to reach US$ 12.6 billion by 2036, expanding at a compound annual growth rate (CAGR) of 4.7% from 2026 to 2036. This steady growth trajectory reflects the essential and non-substitutable role of anesthesia drugs in modern healthcare systems. As surgical interventions continue to rise globally-across both elective and emergency procedures-the demand for safe, effective,…
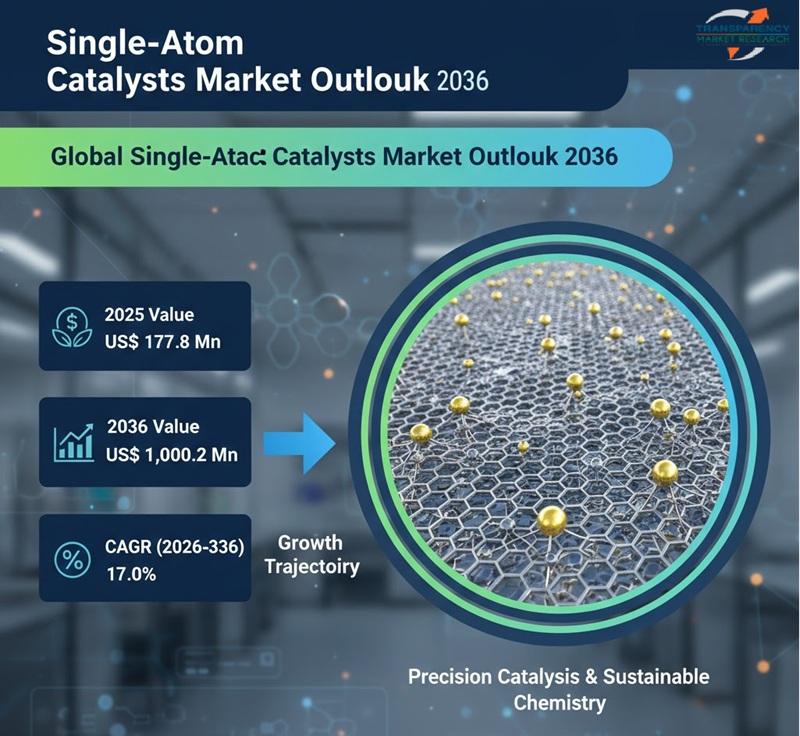
Single-Atom Catalysts Market Size is Expected to Expand from US$ 177.8 Million t …
The global single-atom catalysts (SACs) market is poised for remarkable growth as industries seek highly efficient, cost-effective, and sustainable catalytic solutions. Valued at US$ 177.8 million in 2025, the market is projected to reach US$ 1,000.2 million by 2036, expanding at a robust compound annual growth rate (CAGR) of 17.0% from 2026 to 2036. This rapid expansion reflects the growing importance of advanced catalysis in energy, chemicals, environmental protection, and…
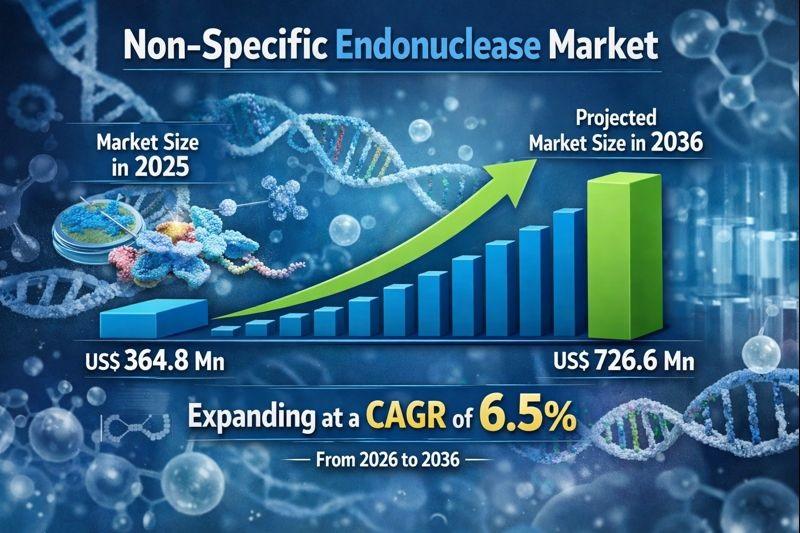
Non-specific Endonuclease Market to Reach USD 726.6 Million by 2036, Supported b …
The non-specific endonuclease market is witnessing steady growth, driven by the expanding use of molecular biology tools across biotechnology, pharmaceuticals, diagnostics, and academic research. Non-specific endonucleases are enzymes that cleave nucleic acids without requiring a specific recognition sequence, making them highly valuable for applications such as DNA/RNA degradation, sample preparation, viscosity reduction, and contamination control. Their broad activity profile differentiates them from restriction enzymes and enables versatile usage across multiple…
More Releases for RNA
CD Formulation Launches Custom Circular RNA Synthesis Service to Accelerate RNA …
CD Formulation introduces a customizable circRNA synthesis service, delivering high-quality, stable circRNAs for therapeutics, vaccines, and gene research, supported by advanced design and QC processes.
CD Formulation, a leading provider of advanced small nucleic acid synthesis [https://www.formulationbio.com/nucleic-acid/custom-small-nucleic-acid-synthesis.html] solutions, is proud to announce the launch of its fully customizable circular RNA (circRNA) synthesis service. This new service addresses the growing need for stable, non-immunogenic RNA molecules for therapeutic development, vaccine research, and…
Self-Amplifying RNA Synthesis Market Gains Traction as Biotech Firms Embrace Sca …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " Self-Amplifying RNA Synthesis Market- (By Product & Service (Products (Enzymes & Reagents, Premade saRNA, Others), Custom Synthesis Services), By Application (Therapeutics Development (Oncology, Infectious Diseases, Others), Biomedical Research), By End-User (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic,…
RNA Extraction and RNA Purification Market: Growth, Trends & Competitive Landsca …
The global RNA Extraction and RNA Purification Market is expected to grow at 6.3% CAGR from 2025 to 2032.
This Market Report is the result of extensive research and analysis conducted by our team of experienced market researchers through -
• 70% efforts of Primary Research
• 15% efforts of Secondary Research
• 15% efforts from the subscription to Paid database providing industry overview, macro and micro economics factors, and financials of private limited…
RNA Targeting Small Molecules Therapeutics Market: Exponential Growth with Risin …
Estimations Predict a CAGR of 29.8% by 2029 in Global RNA Targeting Small Molecules Therapeutics Market Boosted by Precision Medicine, RNA Biomarker Identification and RNA Genetic Manipulation
What Is The Projected Market Size of The Global RNA Targeting Small Molecules Therapeutics Market And Its Growth Rate?
• The market will grow from $6.1 billion in 2024 to $7.87 billion in 2025 at a compound annual growth rate (CAGR) of 28.9%.
• Expected exponential…
Global DNARNA Extraction Kit Market by Type (Cell-free DNA (cfDNA), Sequence-spe …
"DNARNA Extraction Kit Market" is segmented by Company, Region (country), By Type, Application, stakeholders and other participants. This report provides an analysis of revenue and forecast across Type and Application segments for 2023-2032.
The market for DNARNA Extraction Kits has been thoroughly researched via primary and secondary sources to produce this research study. Along with a competitive analysis of the market, segmented by application, type, and geographical trends, it offers a…
Cancer RNA Expression Market to Reap Excessive Revenues by 2028(By sequencing te …
Worldwide cancer is one of the leading cause of death and effective way of treating it still looks unaccomplished in most parts of the world. The factors which influence the successful treatment of cancer are different depending on the stage of diagnosis, treatment availability and availability of trained healthcare professionals coupled with high economic burden of the disease. The gene expression of cancerous cells varies by cancer type and may…
