Press release
Antibody Testing Market to Hit US$16 Bn by 2032, Reports Persistence Market Research
The antibody testing market is entering a transformative decade, shaped by rising infectious disease prevalence, rapid innovation in immunodiagnostics, and increasing integration with digital health technologies. From pandemic preparedness to precision oncology, antibody-based diagnostics continue to evolve as essential tools for clinicians, researchers, and public health agencies worldwide. As diagnostic technologies mature and healthcare ecosystems shift toward preventive and personalized medicine, antibody testing has become central to disease surveillance, treatment decision-making, and therapeutic monitoring. This article offers a comprehensive, fully original analysis of the market, covering growth drivers, restraints, opportunities, segmentation, company insights, regional developments, and recent strategic movements influencing the industry's trajectory.Get a Sample Copy of Research Report (Use Corporate Mail id for Quick Response): https://www.persistencemarketresearch.com/samples/31930
Market Overview and Growth Outlook
The global antibody testing market is poised for steady and sustained expansion, underpinned by growing healthcare awareness, rising investments in public health systems, and an intensifying need for accurate diagnostic solutions. The market is projected to reach US$9.4 billion in 2025, with a strong outlook toward US$16 billion by 2032, progressing at a CAGR of 7.8% during the forecast period. This growth is reinforced by steady improvements in healthcare infrastructure, increasing availability of advanced immunoassay platforms, and the diversification of antibody testing applications across infectious diseases, oncology, autoimmune conditions, and vaccine monitoring.
Increasing disease burden remains one of the strongest catalysts supporting adoption. Chronic conditions, respiratory diseases, and emerging viral threats have collectively heightened the demand for reliable serological testing and antibody-based diagnostics. Governments and health institutions place greater emphasis on population-level surveillance, while research grants encourage innovation in high-sensitivity assays, multiplexing capabilities, and remote diagnostics. As a result, antibody testing has moved beyond traditional laboratory-based use cases and is increasingly incorporated into home-based health models, community screenings, and telemedicine-supported workflows.
From a segmental standpoint, ELISA-based assays are expected to dominate as the leading segment in 2025, accounting for approximately 44% of total market share due to their precision, cost-effectiveness, and broad clinical adoption. Regionally, North America will maintain its leadership position with a 39% share in 2025, supported by sophisticated healthcare systems, favorable reimbursement structures, and consistent technological innovation. Simultaneously, the Asia Pacific region is projected to experience the fastest growth, fueled by rapid urbanization, expanding diagnostic laboratory networks, and growing acceptance of point-of-care testing solutions.
Key Highlights from the Report
• Global market value expected to reach US$16 billion by 2032, expanding at a CAGR of 7.8%.
• North America projected to lead with 39% market share in 2025, while Asia Pacific is the fastest-growing region.
• Diagnostic laboratories to dominate end-user share at 37% in 2025, with research institutes growing the fastest.
• ELISA-based assays to maintain a leading 44% share in 2025; rapid lateral flow assays to grow the quickest.
• July 2025: Agilus Diagnostics and Sebia launch Anti-MCV test for early rheumatoid arthritis detection.
• Strategic emphasis on rapid diagnostics, next-generation assay development, and partnerships with global hospital networks.
Market Segmentation
Product and Assay Type Analysis
Antibody testing comprises a diverse range of assay technologies, each designed to offer varying levels of sensitivity, throughput, and usability depending on clinical or research needs. ELISA assays continue to command the largest share of the market due to their unparalleled accuracy, strong regulatory acceptance, and wide spectrum of applications. Their compatibility with automated analyzers and high-throughput lab workflows makes them indispensable for hospitals and diagnostic centers. Several decades of clinical validation have resulted in optimized protocols and robust manufacturing pipelines, strengthening ELISA's position within core diagnostic portfolios worldwide.
In contrast, rapid lateral flow assays represent the most dynamic and fastest-growing segment of the market. Growing interest in point-of-care diagnostics-especially in low-resource regions-has accelerated adoption of lateral flow tests, which deliver rapid, user-friendly results without requiring complex laboratory instrumentation. Recent advances in microfluidics, advanced biosensors, and smartphone-assisted interpretation technologies are pushing these assays into broader use cases, from emergency medical departments to community health campaigns and at-home diagnostic kits. Furthermore, multiplex designs capable of detecting several antibodies simultaneously are redefining rapid assay capabilities and strengthening their clinical value.
Application-Based Segmentation
The antibody testing market demonstrates substantial application diversity, driven by evolving healthcare needs and expanded use cases across medical specialties. Infectious disease testing remains the dominant application, accounting for an estimated 51% share in 2025. Ongoing disease surveillance, preparedness initiatives, and high incidence of chronic viral infections sustain strong adoption within this segment. Increasing global mobility and the rising threat of zoonotic outbreaks underscore the need for reliable serological testing to support containment strategies and confirm infection exposure.
Meanwhile, oncology-focused antibody testing is emerging as the fastest-growing application. Modern cancer care increasingly depends on antibody-based diagnostics to identify biomarkers, evaluate immune responses, and guide patient-specific treatment plans. With rapid advancements in immunotherapy, companion diagnostics have become essential in tailoring interventions, predicting therapy response, and monitoring disease progression. Hospitals and research institutes continue to integrate advanced antibody assays into oncology workflows, supported by funding for precision medicine initiatives worldwide.
End-User Segmentation
End-user adoption patterns reflect the expanding utility of antibody diagnostics across healthcare and research ecosystems. Diagnostic laboratories represent the largest end-user segment, maintaining around 37% market share due to their established infrastructure, access to automated systems, and skilled laboratory personnel. Their centralized operations support cost-efficient processing of large patient sample volumes, making them critical hubs for ongoing disease surveillance, vaccine monitoring, and routine screening programs.
Conversely, academic and research institutions are forecast to be the fastest-growing end-user group as global investments in research intensify. Universities, biotech companies, and research consortia increasingly rely on cutting-edge antibody technologies for biomarker discovery, immunotherapy development, and population-level epidemiological studies. The adoption of advanced platforms-such as digital ELISA, high-sensitivity chemiluminescent assays, and proteomics-enabled antibody profiling-is significantly higher in research settings, reflecting the complex, experimental nature of their applications.
Read Detailed Analysis: https://www.persistencemarketresearch.com/market-research/antibody-testing-market.asp
Regional Insights
North America
North America will remain the dominant regional market through 2025, with the United States contributing a significant portion of the region's 39% share. The region's leadership is anchored by strong healthcare spending, widespread deployment of advanced immunodiagnostic platforms, and extensive research activities backed by public and private sector funding. The presence of major diagnostic manufacturers ensures rapid adoption of technologically advanced products, while robust regulatory frameworks-such as FDA guidance on diagnostic accuracy-support consistent quality standards. Investments in AI-based diagnostic automation, telemedicine integration, and personalized medicine programs continue to stimulate market expansion.
Europe
Europe represents the second-largest regional market, projected to capture 27% of global share in 2025. Germany, France, and the United Kingdom drive the region's growth through their strong biotechnology ecosystems and national priorities around early disease detection. EU-wide initiatives such as the In Vitro Diagnostic Regulation (IVDR) create harmonized quality standards and promote diagnostic transparency, while Horizon Europe research funding fosters innovation and interdisciplinary collaboration. European demand increasingly favors eco-friendly testing solutions, data-integrated diagnostic tools, and standardized assay platforms suited for cross-border research networks.
Asia Pacific
The Asia Pacific region is expected to experience the fastest growth rate through 2032 due to rising healthcare investments, increasing prevalence of infectious diseases, and expanding access to diagnostic services. China's Healthy China 2030 initiative supports large-scale screening programs, significantly boosting demand for antibody tests. Japan and South Korea contribute to high-end technology adoption, while India and ASEAN countries prioritize affordability and access through expanded public health programs. Rapid urbanization, rising incomes, and growing awareness of preventive healthcare are accelerating market expansion, making Asia Pacific the most strategic region for both multinational companies and domestic manufacturers.
Market Drivers
The antibody testing market benefits from several structural and clinical drivers. Growing global disease burdens-including respiratory infections, chronic illnesses, and emerging viral threats-significantly elevate the use of antibody-based diagnostics. The WHO's reports on rising mortality from respiratory infections and the long-term impact of COVID-19 have reinforced the importance of scalable diagnostic infrastructure. Additionally, expanding point-of-care capabilities and the shift toward decentralized health models support rapid test adoption across remote and underserved regions.
Technological innovation forms another major growth catalyst. Advances in immunodiagnostics-such as digital ELISA, automated chemiluminescence systems, and multiplex assays-are improving diagnostic accuracy, while AI and machine learning optimize laboratory workflows and reduce human error. With nearly one-third of new diagnostic products focusing on antibody testing improvements, manufacturers that invest in next-generation platforms are set to capture high-value market segments in research and clinical applications.
Market Restraints
Despite strong momentum, the market faces technical and regulatory challenges. Cross-reactivity issues continue to undermine test accuracy, particularly in autoimmune conditions and viral infections. Studies revealing significant false-positive risks highlight the need for meticulous validation and standardized epitope mapping. These scientific complexities often extend development timelines and increase R&D expenses.
Regulatory inconsistencies also present barriers. Global agencies-including the FDA, EMA, and national regulatory bodies-impose varying validation requirements, complicating cross-market approvals and inflating compliance costs. The lack of universally accepted diagnostic standards contributes to research irreproducibility and delays product launches, posing significant hurdles for emerging companies and innovators.
Market Opportunities
The integration of antibody testing into remote healthcare ecosystems represents one of the most promising areas of future expansion. Home-based diagnostics, enabled by telemedicine, are projected to exceed US$1.2 billion by 2030, driven by consumer demand for convenience, privacy, and real-time clinical interpretation. Subscription-based testing models for chronic care, wellness monitoring, and routine screenings offer sustainable revenue pathways for manufacturers.
Meanwhile, frontier technologies such as AI-driven assay optimization, single-cell proteomics, CRISPR-enabled diagnostics, and mass spectrometry validation are redefining the antibody testing landscape. These innovations enable ultra-sensitive detection, personalized medicine applications, and enhanced diagnostic precision. Emerging economies represent additional high-growth opportunities through expanding laboratory networks, localized production, and cost-effective biosensor technologies that address infrastructure constraints.
Request for Customization of the Research Report: https://www.persistencemarketresearch.com/request-customization/31930
Company Insights
• Roche Holding AG
• Abbott Laboratories
• Thermo Fisher Scientific Inc.
• Siemens Healthineers AG
• Bio-Rad Laboratories Inc.
• Danaher Corporation
• Becton, Dickinson and Company
• Merck KGaA
• PerkinElmer Inc.
• Eurofins Scientific SE
• Agilent Technologies Inc.
• QuidelOrtho Corporation
• DiaSorin S.p.A.
• Luminex Corporation
• Hologic Inc.
Recent Developments
In October 2025, Boster Biological Technology launched a free antibody validation program, strengthening research reproducibility and expanding its IHC service portfolio.
In June 2025, SIAT and the Chinese Academy of Sciences developed a compact fingertip blood-based COVID-19 antibody testing platform for point-of-care and at-home applications.
Conclusion
The antibody testing market is on a robust growth trajectory driven by persistent infectious disease challenges, rapid advancements in diagnostic technologies, and the global shift toward precision medicine. As governments strengthen disease surveillance systems and healthcare providers adopt more sophisticated diagnostic tools, antibody testing will remain central to both clinical decision-making and public health strategy. While regulatory complexities and accuracy limitations pose challenges, the industry's sustained innovation in digital diagnostics, home-based testing, and next-generation assay platforms promises significant future opportunities. By aligning with evolving healthcare needs and expanding capabilities, the antibody testing market is well-positioned to deliver impactful, high-value diagnostic solutions throughout the coming decade.
Read More Related Reports:
Antibody Testing Market https://www.persistencemarketresearch.com/market-research/antibody-testing-market.asp
Healthcare Chatbots Market https://www.persistencemarketresearch.com/market-research/healthcare-chatbots-market.asp
Newborn Metabolic Screening Market https://www.persistencemarketresearch.com/market-research/newborn-metabolic-screening-market.asp
Non-muscle Invasive Bladder Cancer Therapeutics Market https://www.persistencemarketresearch.com/market-research/nonmuscle-invasive-bladder-cancer-therapeutics-market.asp
Contact Us:
Persistence Market Research
Second Floor, 150 Fleet Street, London, EC4A 2DQ, United Kingdom
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Antibody Testing Market to Hit US$16 Bn by 2032, Reports Persistence Market Research here
News-ID: 4276890 • Views: …
More Releases from Persistence Market Research
India Aluminum Beverage Can Market Size to Reach US$ 0.8 Bn by 2032 - Persistenc …
The India aluminum beverage can market is undergoing a significant transformation, driven by changing consumer lifestyles, rising urbanization, and a noticeable shift toward sustainable and convenient packaging formats. Aluminum beverage cans are increasingly preferred across carbonated soft drinks, energy drinks, sports beverages, alcoholic drinks, and ready-to-drink juices due to their lightweight structure, portability, fast chilling properties, and superior recyclability. In India, where on-the-go consumption is accelerating rapidly, aluminum cans are…
Medical Devices Packaging Market Size to Reach US$ 41.57 Billion by 2032 - Persi …
The medical devices packaging market plays a vital role within the global healthcare ecosystem, acting as a protective and regulatory bridge between manufacturers and end users. Medical device packaging refers to specialized materials and formats designed to safeguard medical instruments, implants, diagnostic tools, and consumables throughout storage, transportation, and clinical use. These packaging solutions are engineered to maintain sterility, prevent contamination, ensure ease of handling, and comply with strict regulatory…
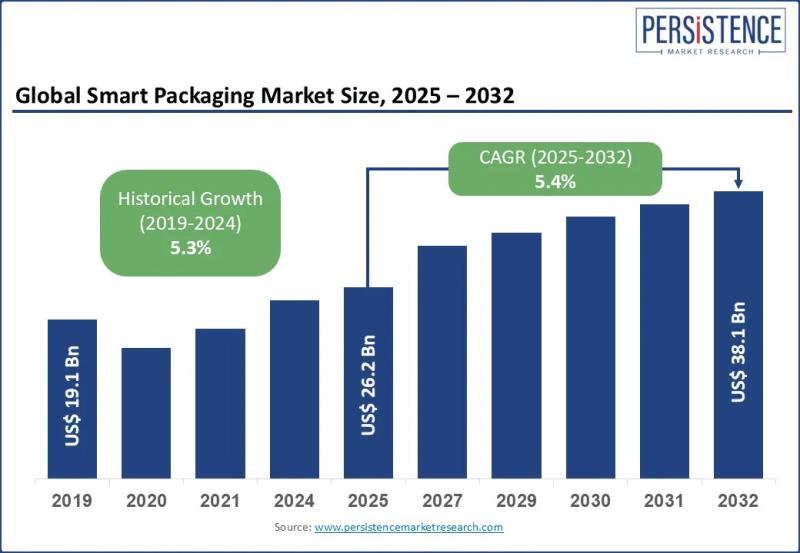
Smart Packaging Market Size Valued at US$ 26.2 Bn in 2025, Projected to Reach US …
The smart packaging market is rapidly transforming the global packaging landscape by integrating advanced technologies with traditional packaging materials to deliver enhanced functionality, traceability, and consumer engagement. Smart packaging refers to packaging systems embedded with features such as sensors indicators QR codes RFID tags and data tracking mechanisms that monitor product condition authenticity and movement across the supply chain. These solutions are increasingly adopted as businesses shift from passive containment…

Football Equipment Market Set for Strong Global Growth Through 2032
The global football equipment market continues to display resilient growth driven by rising participation in football across all age groups, expanding commercial opportunities, and technological advancements in sports gear. The industry is expected to grow from an estimated US$ 18.7 billion in 2025 to approximately US$ 24.1 billion by 2032, registering a compound annual growth rate (CAGR) of 3.7% over the forecast period.
➤ Download Your Free Sample & Explore Key…
More Releases for Antibody
OBP2A Antibody Market Thrives on Technological Innovations in Antibody Productio …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global OBP2A Antibody Market- (By Type (Polyclonal Antibodies, and Monoclonal Antibodies; By Application-(Enzyme Linked Immunosorbent Assay, Immunohistochemistry, & Western Blot)), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global OBP2A Antibody Market is valued at US$ 102.9 Mn in 2023, and it is expected to…
Evolving Market Trends In The Antibody Drug Conjugates Industry: Regulation On A …
The Antibody Drug Conjugates Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Expected Antibody Drug Conjugates Market Size During the Forecast Period?
The market size for antibody drug conjugates has experienced rapid expansion in the past few years. It's projected to rise from…
TIGIT Antibody Clinical Trials FDA Approval Market Size Anti TGIT Antibody Sales …
Anti TIGIT Antibodies Clinical Trials & Market Opportunity Outlook 2028 Report Highlights:
• Anti TIGIT Antibodies In Clinical Trials: > 50 Antibodies
• First Anti TIGIT Antibody To Get Approval Within Next 5 Years
• Global Anti TIGIT Antibodies Clinical Pipeline Insight By Company, Indication and Phase
• Insight On More Than 50 Anti TIGIT Antibodies In Clinical Trials
• Anti TIGIT Antibodies Market Trends by Indication & Country
• Global Anti TIGIT Antibodies Market Dynamics
Download Report:
https://www.kuickresearch.com/report-anti-tigit-antibody-anti-tigit-antibodies-fda-approved-tigit-antibody-tigit-inhibitors-tigit-drugs-approved-tigit-expression-tigit-ligand-tigit-gene
In recent years, the global…
Mammalian Polyclonal IgG Antibody Market, Mammalian Polyclonal IgG Antibody Mark …
"According to the research report, the global inspection machines market was valued at USD 845.21 million in 2022 and is expected to reach USD 1,437.59 million by 2032, to grow at a CAGR of 5.5% during the forecast period."
Request Our Free Sample Report for Inspection Machines Market Insights and Emerging Trends @ https://www.polarismarketresearch.com/industry-analysis/inspection-machines-market/request-for-sample
Report Overview
Polaris Market Research, a leading global market research and consulting company, has recently published its latest report…
Global Combination Antibody Therapy Market 2022: Antibody/Antibody Combination S …
The combination antibody therapy market is expected to experience significant growth in the coming years. Combination antibody therapy involves the use of two or more monoclonal antibodies (mAbs) in combination to treat various diseases, such as cancer, autoimmune disorders, and infectious diseases.
Request For Free Sample Report of "Combination Antibody Therapy Market"@ https://www.persistencemarketresearch.com/samples/11740
Combination antibody therapy has several potential benefits over single-agent therapies, including increased efficacy, improved patient outcomes, and reduced toxicity.…
Global Combination Antibody Therapy Market 2022: Antibody/Antibody Combination S …
Combination antibody therapy involves the use of two or more antibodies to target and treat a specific disease or condition. This type of therapy is often used in cancer treatment, as it can be more effective at targeting and killing cancer cells than single-antibody therapies. Combination antibody therapy can also be used to treat a range of other diseases and conditions, including autoimmune diseases, infectious diseases, and allergic conditions.
Request For…
