Press release
Biologics Contract Manufacturing Market Driven by Rising Biopharma Outsourcing: Persistence Market Research
The biologics contract manufacturing market has emerged as one of the fastest-expanding pillars of the global biopharmaceutical ecosystem. Driven by the surging demand for complex biologics-from monoclonal antibodies to recombinant proteins and cell-based therapies-pharmaceutical and biotech innovators are increasingly turning to Contract Development and Manufacturing Organizations (CDMOs) to augment their production capacity, accelerate commercialization, and reduce operational costs. With biologics becoming central to modern therapeutic portfolios, outsourcing continues to gain strategic significance. According to industry assessments, the market generated US$ 14.4 billion in 2022 and is projected to reach US$ 42.1 billion by 2033, expanding at a robust 8.6% CAGR throughout the forecast period.Get a Sample Copy of Research Report (Use Corporate Mail id for Quick Response): https://www.persistencemarketresearch.com/samples/31928
Market Overview and Key Growth Trends
The biologics contract manufacturing landscape is shaped by rapid technological advancements, chronic disease burden, and the rising complexity of therapeutic development cycles. Modern biopharmaceutical companies seek specialized partners capable of providing end-to-end services-from cell-line development and upstream processing to fill-finish operations. As biologics pipelines continue to expand, with over 1,500 biomolecules currently in clinical trials, contract manufacturers are witnessing unprecedented demand for flexible, efficient, and high-quality production platforms.
A major factor influencing market expansion is the dominance of mammalian expression systems, which accounted for 72.6% of the global market in 2022. These platforms offer superior compatibility with human proteins and ensure a high degree of product stability, making them ideal for monoclonal antibody and vaccine manufacturing. Geographically, North America leads the global market, with the United States capturing nearly 89.9% of the regional share, backed by cutting-edge R&D infrastructures, high venture capital inflow, and a well-established network of biopharmaceutical developers. This leadership is complemented by strong regional demand for biologics and progressive adoption of outsourcing models.
Key Highlights from the Report
• Global market revenue reached US$ 14.4 billion in 2022 and is projected to hit US$ 42.1 billion by 2033.
• Mammalian biologics platforms accounted for 72.6% of total industry share in 2022.
• Monoclonal antibodies represented the largest product segment with a 40.7% market share.
• Oncology led therapeutic-area demand with 24.8% market contribution in 2022.
• Commercial-scale biologics manufacturing held a dominant 71.4% revenue share.
• The top five countries collectively contributed 67.6% of overall market valuation.
Market Segmentation
Segmentation by Product Type
The biologics contract manufacturing market includes a diverse set of products, ranging from monoclonal antibodies and recombinant proteins to vaccines, plasmids, and advanced therapeutics. Monoclonal antibodies (mAbs) remain the most prominent segment, capturing 40.7% of the market in 2022 due to their exceptional clinical specificity and proven therapeutic value. The growing adoption of bispecific antibodies, antibody-drug conjugates (ADCs), and emerging mAb derivatives further augments the need for specialized contract manufacturing expertise. Recombinant proteins and vaccines also represent substantial portions of the market, driven by increasing vaccine demand and the global expansion of targeted therapeutic research.
Segmentation by Platform
Manufacturing platforms continue to be dominated by mammalian cell lines, especially CHO (Chinese Hamster Ovary) cells. The mammalian segment held 72.6% share in 2022, growing at a projected 9.2% CAGR. This platform's ability to generate biologically relevant proteins with appropriate post-translational modifications makes it crucial for producing high-quality therapeutic proteins and monoclonal antibodies. Microbial expression platforms also remain important for smaller proteins and enzymes, while cell-free and novel expression systems are gaining traction for rapid prototyping and next-generation biologics.
Segmentation by Therapeutic Area
Oncology continues to drive the largest portion of biologics demand, representing 24.8% of total market revenues. The increasing global burden of cancer, combined with breakthroughs in immuno-oncology, has intensified the need for advanced biologics manufacturing services. Other key therapeutic areas include autoimmune diseases, infectious diseases, metabolic disorders, and rare diseases. Clinical research activity is particularly intense in personalized medicine and gene-modified therapies, creating new avenues for CDMOs specializing in niche segments.
Segmentation by Application
The biologics contract manufacturing market is divided into clinical and commercial applications, with commercial manufacturing accounting for 71.4% of total revenues in 2022. As more biologic drugs gain regulatory approval and enter large-scale production phases, CDMOs play a critical role in ensuring capacity scalability, technology transfer, and regulatory compliance. Clinical manufacturing, however, remains essential in supporting early-stage biopharma innovators through development, optimization, and trial-phase supply.
Read Detailed Analysis: https://www.persistencemarketresearch.com/market-research/biologics-contract-manufacturing-demand.asp
Regional Insights
North America stands at the forefront of the biologics contract manufacturing industry. The United States commands a striking majority of the regional market due to its extensive biotechnology ecosystem, supportive regulatory framework, and high concentration of pharmaceutical giants. R&D investments remain significant, and biologics pipelines continue to progress rapidly, contributing to the rising adoption of CDMO partnerships.
Europe forms another major hub, with Germany contributing 33.7% of the regional market share. The region's strong focus on next-generation biologics, stringent regulatory standards, and increasing clinical trial activities drive outsourcing demand. Furthermore, European companies are increasingly adopting flexible and integrated solutions offered by CDMOs to maintain competitiveness in a rapidly shifting therapeutic landscape.
In Asia-Pacific, China has emerged as a powerful growth engine, holding 18.2% of the East Asian market. The country's strategic push in biopharma investment, skilled workforce expansion, and collaborations between global and local manufacturers contribute to its rising prominence. Additionally, regional governments are promoting biotechnology innovation, creating an attractive environment for CDMO expansion. Other emerging markets including India, South Korea, and Singapore are also increasing their investments in biologics R&D and manufacturing infrastructure.
Market Drivers
Market growth is propelled by the expanding pipeline of biologics, technological advancements in bioprocessing, and increased reliance on outsourcing due to capacity limitations in pharmaceutical companies. With over 1,500 biologics in clinical trials, developers increasingly require partners to support process development, scale-up, and GMP production. The rising prevalence of chronic diseases, especially cancer and autoimmune disorders, accelerates demand for targeted biologics that require specialized manufacturing environments.
Technological innovations such as single-use bioreactors, integrated continuous manufacturing, and automation are enhancing production flexibility and reducing operational costs. CDMOs are adopting advanced platforms to streamline workflows, increase batch consistency, and shorten timelines. Moreover, the expiration of key biologic patents and the rapid uptake of biosimilars create additional demand for high-volume, cost-effective manufacturing solutions. As large pharmaceutical companies prioritize pipeline diversification and speed to market, the outsourcing model continues to serve as a strategic tool for maintaining efficiency and competitiveness.
Market Restraints
Despite strong market momentum, several challenges hinder industry expansion. One significant restraint stems from regulatory complexities associated with biologics manufacturing. Global regulatory frameworks involving cGMP standards, quality audits, and cross-border supply chain oversight increase operational costs and create barriers to entry for smaller CDMOs. Compliance with stringent guidelines can be resource-intensive and requires continuous technological upgrades.
Additionally, the biologics sector faces a persistent shortage of skilled professionals in process engineering, analytical development, and quality assurance. Many emerging markets struggle with insufficient training infrastructure, resulting in a talent gap that impacts the scalability of biologics manufacturing. Financial constraints, inadequate biotech funding, and high initial capital requirements further limit the ability of smaller firms to compete in a market dominated by global giants.
Market Opportunities
Significant opportunities are arising from the expansion of biosimilar pipelines, growing demand for personalized medicine, and the emergence of advanced therapies such as gene and cell therapies. As biologics patents continue to expire, CDMOs are well-positioned to support biopharma companies in developing cost-competitive biosimilars for large patient populations. Additionally, next-generation platforms such as CAR-T therapies and nucleic acid-based therapeutics require specialized manufacturing capabilities, opening new revenue streams.
CDMOs are increasingly embracing integrated service models that combine development, manufacturing, and regulatory support, providing seamless end-to-end solutions for clients. This integration enhances efficiency, reduces time-to-market, and strengthens client relationships. Geographic expansion into emerging markets also presents growth potential, with multinational CDMOs establishing facilities to cater to local demands and leverage cost advantages. Collectively, these opportunities promise to reshape the competitive landscape and unlock long-term market value.
Request for Customization of the Research Report: https://www.persistencemarketresearch.com/request-customization/31928
Company Insights
Key Players
• Samsung Biologics
• BioXcellence (Boehringer Ingelheim)
• Lonza Group AG
• Fujifilm Diosynth Biotechnologies
• AbbVie CM (AbbVie Inc.)
• WuXi Biologics (Cayman) Inc.
• AGC Biologics
• Patheon N.V. (Thermo Fisher Scientific Inc.)
• Emergent BioSolutions Inc.
• Ajinomoto Bio-Pharma
• Avid Bioservices, Inc.
• KBI Biopharma
• Rentschler Biotechnologie GmbH
• Merck KGaA
• Catalent Inc.
• Therapure Biopharma Inc.
• Novasep
• Abzena plc.
• ProBioGen AG
Key Segments Covered in Biologics Contract Manufacturing Industry Research
Product:
Monoclonal Antibodies
Recombinant Proteins
Vaccines
Insulin
Interferons
Growth Factors
Others
Platform:
Mammalian
Microbial
Therapeutic Area:
Oncology
Autoimmune Disease
Metabolic Disease
Ophthalmology
Cardiovascular Disease
Infectious Disease
Neurology
Respiratory Disorder
Others
Application:
Commercial
Clinical
Region:
North America
Latin America
Europe
South Asia
East Asia
Oceania
Middle East & Africa
Recent Developments
Lonza Group AG achieved a major strategic milestone by selling its Lonza Specialty Ingredients division to Bain Capital Private Equity in 2021, enabling intensified focus on biologics manufacturing capabilities.
Abzena Ltd. collaborated with BiVictriX Therapeutics in 2021 to support the manufacturing of next-generation ADCs, highlighting the growing importance of targeted bioconjugate therapies.
Conclusion
The biologics contract manufacturing market is entering a period of sustained expansion, supported by the growing complexity of biologics pipelines, increasing reliance on outsourcing, and continued technological innovation. As biopharmaceutical companies strive to accelerate development timelines and meet global therapeutic demands, CDMOs will remain indispensable partners in the commercialization journey. With a projected market valuation of US$ 42.1 billion by 2033, the sector promises robust opportunities for established players and emerging innovators alike. Strategic collaborations, investment in high-potency biomanufacturing, and adoption of cutting-edge bioprocessing tools will define the next decade of market leadership and scientific advancement.
Read More Related Reports:
Cellular Health Screening Market https://www.persistencemarketresearch.com/market-research/cellular-health-screening-market.asp
Pelvic Organ Prolapse Repair Market https://www.persistencemarketresearch.com/market-research/pelvic-organ-prolapse-repair-market.asp
Allergy Shots Market https://www.persistencemarketresearch.com/market-research/allergy-shots-market.asp
Biologics Contract Manufacturing Market https://www.persistencemarketresearch.com/market-research/biologics-contract-manufacturing-demand.asp
Contact Us:
Persistence Market Research
Second Floor, 150 Fleet Street, London, EC4A 2DQ, United Kingdom
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biologics Contract Manufacturing Market Driven by Rising Biopharma Outsourcing: Persistence Market Research here
News-ID: 4276878 • Views: …
More Releases from Persistence Market Research

Mining Waste Management Market on Track to Reach US$368.9 Billion by 2033 Driven …
Mining Waste Management Market Overview and Growth Outlook
The mining waste management market is emerging as a critical pillar of the global mining value chain, driven by rising environmental scrutiny and the growing scale of mining operations worldwide. According to the latest study by Persistence Market Research, the global mining waste management market size is likely to be valued at US$262.2 billion in 2026 and is expected to reach US$368.9 billion…
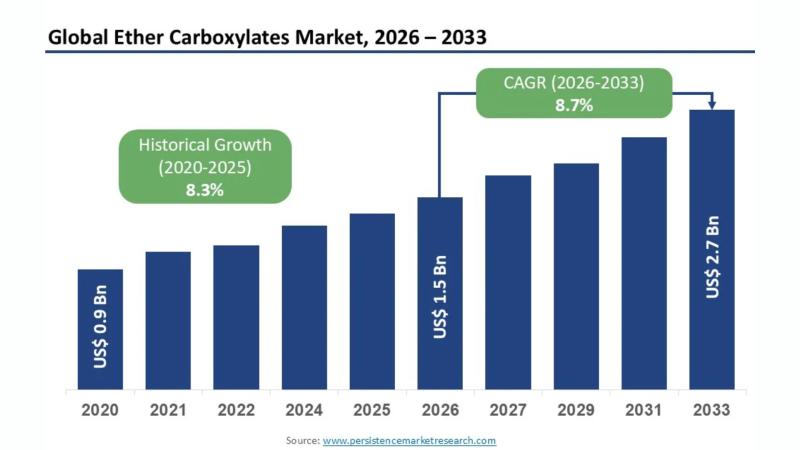
Ether Carboxylates Market Predicted to Reach US$2.7 Billion by 2033 Driven by Su …
Ether Carboxylates Market Overview and Growth Outlook
The ether carboxylates market is witnessing robust expansion as industries increasingly adopt high-performance, environmentally compatible surfactants. According to the latest study by Persistence Market Research, the global ether carboxylates market size is likely to be valued at US$1.5 billion in 2026 and is expected to reach US$2.7 billion by 2033, growing at a strong CAGR of 8.7% during the forecast period from 2026 to…

Epoxidized Soybean Oil Market Forecasted to Hit US$1.8 Billion by 2033 Driven by …
Epoxidized Soybean Oil Market Overview and Growth Outlook
The epoxidized soybean oil market is gaining strong momentum as industries increasingly prioritize sustainability, safety, and regulatory compliance. According to the latest study by Persistence Market Research, the global epoxidized soybean oil market size is likely to be valued at US$1.3 billion in 2026 and is expected to reach US$1.8 billion by 2033, expanding at a CAGR of 4.8% during the forecast period…

EV Charging Infrastructure Market to Reach US$ 113.4 Bn by 2032 as Key Players T …
The global electric vehicle (EV) charging infrastructure market is poised for unprecedented growth in the coming decade. Valued at an estimated US$31.1 billion in 2025, the market is projected to reach US$113.4 billion by 2032, reflecting a robust compound annual growth rate (CAGR) of 20.3% during the forecast period from 2025 to 2032. The rapid adoption of electric vehicles worldwide, combined with significant government-backed initiatives and investments in sustainable mobility,…
More Releases for CDMO
FDP CDMO Research: China FDP CDMO market size is projected to reach USD 1.33 bil …
QY Research Inc. (Global Market Report Research Publisher) announces the release of 2025 latest report "Fraud Detection and Prevention (FDP) System- Global Market Share and Ranking, Overall Sales and Demand Forecast 2025-2031". Based on current situation and impact historical analysis (2020-2024) and forecast calculations (2025-2031), this report provides a comprehensive analysis of the global Wire Drawing Dies market, including market size, share, demand, industry development status, and forecasts for the…
Global Cmo And Cdmo Biotechnology Market Size by Application, Type, and Geograph …
According to Market Research Intellect, the global Cmo And Cdmo Biotechnology market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
Biologics and sophisticated medicines are driving the biotechnology industry for Contract Manufacturing Organizations (CMO) and Contract…
Evolving Market Trends In The Inhalation CDMO Industry: Strategic Collaborations …
The Inhalation CDMO Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Expected Inhalation CDMO Market Size During the Forecast Period?
In recent times, the inhalation CDMO market has experienced significant growth. The market value is expected to increase from $2.08 billion in 2024…
What's Driving the Inhalation CDMO Market 2025-2034: Rising Respiratory Disorder …
How Is the Chondroplasty Market Projected to Grow, and What Is Its Market Size?
The chondroplasty market has seen strong growth in recent years. It will increase from $13.77 billion in 2024 to $14.68 billion in 2025 at a CAGR of 6.5%. This growth is attributed to the rise in sports-related injuries, patient preference for non-total joint replacement procedures, advances in postoperative care, healthcare provider training, and an increasing incidence of…
Lentiviral Vector (LVV) CDMO Services Market Delivering Cures: The Role of LVV C …
Lentiviral Vector (LVV) CDMO Services Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Lentiviral Vector (LVV) CDMO Services Market - (By Type (IIT Grade, IND Grade, Clinical Trial Grade, Commercial Production Grade), By Application (Biopharmaceutical Company, Academic Scientific Research Institution)), Trends, Industry Competition Analysis, Revenue and Forecast To…
Electronic Chemicals CDMO Market Fueling the Electronics Boom: The Rise of the E …
Electronic Chemicals CDMO Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Electronic Chemicals CDMO Market - (By Type (Metals and Pastes, Electronic Specialty Gases, Polymer Compounds, Others), By Application (Battery, Semiconductor, Integrated Circuit, Consumer Electronics, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest…
