Press release
United States Organ-On-Chip Market is expected to reach US$ 940.4 Million by 2032 | Top key players - Emulate, Inc., MIMETAS B.V., Valo Health.
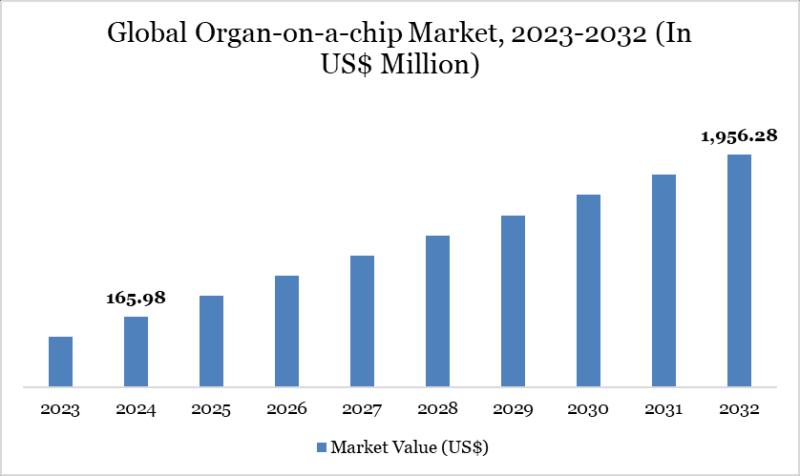
Market Size and Growth:The Global Organ-On-Chip Market Size reached US$ 165.98 million in 2024 and is expected to reach US$ 1,956.28 million by 2032, growing with a CAGR of 36.12% during the forecast period 2025-2032. The Market is growing due to rising demand for advanced drug testing platforms and increasing adoption of microphysiological systems in pharmaceutical and biotech research. According to DataM Intelligence
Get a Free Sample Research PDF: https://datamintelligence.com/download-sample/organ-on-chip-market?sz
The Organ-On-Chip Market involves the development, manufacturing, and application of microfluidic devices that mimic human organ functions for drug testing, disease modeling, and personalized medicine. These chips integrate living cells with sensors to replicate physiological responses, reducing reliance on animal testing, accelerating drug development, and enhancing precision in biomedical research and pharmaceutical testing globally.
Industry Recent Developments: United States
✅ November 2025: Regulatory acceptance from agencies such as the FDA is boosting industry adoption of Organ-On-Chip technology, supported by validation studies demonstrating predictive equivalence with clinical outcomes.
✅ October 2025: The U.S. market is advancing with biotech innovation, and increased FDA validation programs alongside pharmaceutical adoption, making North America the largest market in Organ-On-Chip.
✅ September 2025: Researchers at The Ohio State University developed a "ventilator on a chip" to study lung damage, enabling real-time detection of lung damage at the cellular level.
Industry Recent Developments: Japan
✅ November 2025: Japan maintains dominance in the Asia-Pacific Organ-On-Chip market with a well-established healthcare system focusing on microfluidics, regenerative medicine, and tissue engineering.
✅ October 2025: Japan continues to benefit from growing government support for advancement in healthcare technologies, driving further growth in the Organ-On-Chip market.
✅ September 2025: Japan shows significant efforts in microphysiological systems development with government-backed initiatives and advancements in Organ-On-Chip technology, reflecting the country's focus on innovation in this field.
Get Customization in the report as per your requirements: https://datamintelligence.com/customize/organ-on-chip-market?sz
Key FDA / Regulatory Developments (2025):
FDA Roadmap to Phase Out Animal Testing:
✅ The FDA has laid out a roadmap to reduce reliance on animal testing in preclinical drug development, with organ‐on‐chip systems playing a "central role."
✅ This shift is aligned with broader "new approach methodologies" (NAMs), including microphysiological systems like OoCs and in silico (AI) models.
✅ The move is expected to streamline drug development by allowing submissions based on non-animal-based safety data.
ISTAND Pilot Program - Emulate Liver‐Chip:
✅ Emulate's Liver‐Chip (S1) was accepted into the FDA's ISTAND Pilot Program - making it the first Organ‐Chip to get this distinction.
✅ Through this program, Emulate is working with FDA to qualify the Liver‐Chip for use in IND submissions, particularly for predicting drug-induced liver injury (DILI).
GAO Report & Regulatory Support for NAMs:
✅ A 2025 GAO (Government Accountability Office) report underscores growing regulatory support for non-animal models, including human organ-on-chip systems.
✅ The report signals a shift from exploratory research toward more formal regulatory adoption, easing the pathway for OoC data to be accepted in drug approval processes.
FDA Multi-Year Research Collaboration:
✅ The FDA earlier entered into a multi-year R&D collaboration to evaluate organ‐chip technologies (miniaturized human organ systems on micro-engineered chips) for studying pharmacology, toxicity, disease mechanisms, etc.
Mergers, Acquisitions, and Strategic Partnerships (2025):
OrganOx Acquisition by Terumo:
✅ In a major deal, Terumo (Japanese medical device firm) acquired OrganOx (UK-based) for about US$ 1.5 billion. OrganOx's technology preserves organs (e.g., liver) and is used in transplantation settings.
Emulate - Novo Nordisk Strategic Partnership:
✅ Emulate entered a co-development agreement with Novo Nordisk to build OoC-based assays for diabetes drug discovery and safety profiling.
CN Bio - Pharmaron Partnership:
✅ CN Bio (which makes the PhysioMimix OoC platform) has partnered with Pharmaron to validate its technology and integrate it within Pharmaron's R&D workflows, especially for safety testing and drug development.
Mimetas Expansion:
✅ MIMETAS (OrganoPlate platform provider) is reportedly expanding manufacturing reach via acquisitions to scale up its chip production (according to market‐report sources).
Funding for OrganOx:
✅ Earlier in 2025, OrganOx raised US$ 142 million for U.S. expansion, underscoring strong investor confidence in its OoC‐adjacent technology.
Major Key Players:
1. Emulate, Inc. - Develops organ-on-chip systems that mimic human organ functions for drug testing and disease research, enhancing preclinical testing accuracy.
2. MIMETAS B.V. - Specializes in microfluidic organ-on-chip platforms, offering high-throughput models for pharmaceutical and toxicology studies.
3. Valo Health - Uses AI-driven platforms and organ-on-chip technology to accelerate drug discovery and optimize clinical development.
4. Nortis, Inc. - Provides microfluidic and organ-on-chip solutions for modeling human tissues, focusing on kidney, vascular, and cancer research.
5. AxoSim - Offers human-based nerve-on-chip and cardiac-on-chip systems to support neuropharmacology and cardiac safety testing.
6. BICO - The Bio Convergence Company - Integrates bioprinting, organ-on-chip, and tissue engineering technologies to advance drug development and regenerative medicine.
Segments Covered in the Organ-On-Chip Market:
By Products & ServiceLive: Products, Services.
By Application: Drug Discovery, Toxicology Research, Otherrs.
By End User: Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others.
Regional Analysis for Organ-On-Chip Market:
⇥ North America (U.S., Canada, Mexico)
⇥ Europe (U.K., Italy, Germany, Russia, France, Spain, The Netherlands and Rest of Europe)
⇥ Asia-Pacific (India, Japan, China, South Korea, Australia, Indonesia Rest of Asia Pacific)
⇥ South America (Colombia, Brazil, Argentina, Rest of South America)
⇥ Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of Middle East & Africa)
Buy Now & Get 30% OFF - Grab 50% OFF on 2+ reports: https://www.datamintelligence.com/buy-now-page?report=organ-on-chip-market
Chapter Outline:
⏩ Market Overview: It contains five chapters, as well as information about the research scope, major manufacturers covered, market segments, Organ-On-Chip market segments, study objectives, and years considered.
⏩ Market Landscape: The competition in the Global Organ-On-Chip Market is evaluated here in terms of value, turnover, revenues, and market share by organization, as well as market rate, competitive landscape, and recent developments, transaction, growth, sale, and market shares of top companies.
⏩ Companies Profiles: The global Organ-On-Chip market's leading players are studied based on sales, main products, gross profit margin, revenue, price, and growth production.
⏩ Market Outlook by Region: The report goes through gross margin, sales, income, supply, market share, CAGR, and market size by region in this segment. North America, Europe, Asia Pacific, Middle East & Africa, and South America are among the regions and countries studied in depth in this study.
⏩ Market Segments: It contains the deep research study which interprets how different end-user/application/type segments contribute to the Organ-On-Chip Market.
⏩ Market Forecast: Production Side: In this part of the report, the authors have focused on production and production value forecast, key producers forecast, and production and production value forecast by type.
⏩ Research Findings: This section of the report showcases the findings and analysis of the report.
⏩ Conclusion: This portion of the report is the last section of the report where the conclusion of the research study is provided.
Unlimited Insights. One Subscription: https://www.datamintelligence.com/reports-subscription
Frequently asked questions:
➠ What is the global sales value, production value, consumption value, import and export of Organ-On-Chip market?
➠ Who are the global key manufacturers of the Organ-On-Chip Industry? How is their operating situation (capacity, production, sales, price, cost, gross, and revenue)?
➠ What are the Organ-On-Chip market opportunities and threats faced by the vendors in the global Organ-On-Chip Industry?
➠ Which application/end-user or product type may seek incremental growth prospects? What is the market share of each type and application?
➠ What focused approach and constraints are holding the Organ-On-Chip market?
➠ What are the different sales, marketing, and distribution channels in the global industry?
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release United States Organ-On-Chip Market is expected to reach US$ 940.4 Million by 2032 | Top key players - Emulate, Inc., MIMETAS B.V., Valo Health. here
News-ID: 4276174 • Views: …
More Releases from DataM Intelligence 4Market Research

Optically Clear Adhesive Market Set for Explosive Growth as OLED, Touchscreens, …
Market Overview:
The Global Optically Clear Adhesive Market is expected to grow at a CAGR of 8.5% during the forecast period (2024-2031).
The Optically Clear Adhesive (OCA) Market refers to the global industry focused on transparent bonding materials used to laminate display panels, touchscreens, and optical components in smartphones, tablets, TVs, and automotive displays, offering high light transmission, strong adhesion, low haze, and durability to enhance visual clarity and device performance.
Get a…
Kaposi Sarcoma Market Set for Rapid Growth Amid Rising HIV Cases and Breakthroug …
Market Overview:
The Global Kaposi Sarcoma Market is expected to grow at a CAGR of 5.5% during the forecast period 2024-2031.
The Kaposi Sarcoma Market comprises diagnostics, therapeutics, and supportive care for a rare vascular cancer linked to HHV-8, affecting immunocompromised and aging populations. It includes antiretroviral therapy, chemotherapy, immunotherapy, and emerging targeted treatments, driven by HIV prevalence, organ transplantation, research innovation, regulatory approvals, healthcare access, and growing awareness across developed…

Bullet Proof Glass Market is expected to reach USD 45.0 billion by 2031 | Major …
Market Size and Growth:
The Global Bullet Proof Glass Market size reached USD 15.3 billion in 2022 and is expected to reach USD 45.0 billion by 2031, growing with a CAGR of 16.9% during the forecast period 2024-2031.
The Bullet Proof Glass Market refers to the global industry focused on the production and supply of transparent, impact-resistant glass and laminated composites designed to withstand ballistic threats, explosions, and forced entry. These…

Low-E Glass Market Set to Soar as Energy-Efficient Construction Drives Global De …
Market Overview:
The Global Low-E Glass Market is estimated to grow at a CAGR of 3.3% during the forecast period 2024-2031.
The Low-E Glass Market refers to the global industry producing and supplying low-emissivity coated glazing designed to improve thermal insulation by reflecting infrared heat while transmitting visible light. It is widely used in residential, commercial, and automotive applications to enhance energy efficiency, reduce heating and cooling costs, limit UV penetration,…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…