Press release
Peptide CDMO 2.0 Market Forecast to 2034 with Focus on End-to-End Integrated and Tech-Enabled CDMOs
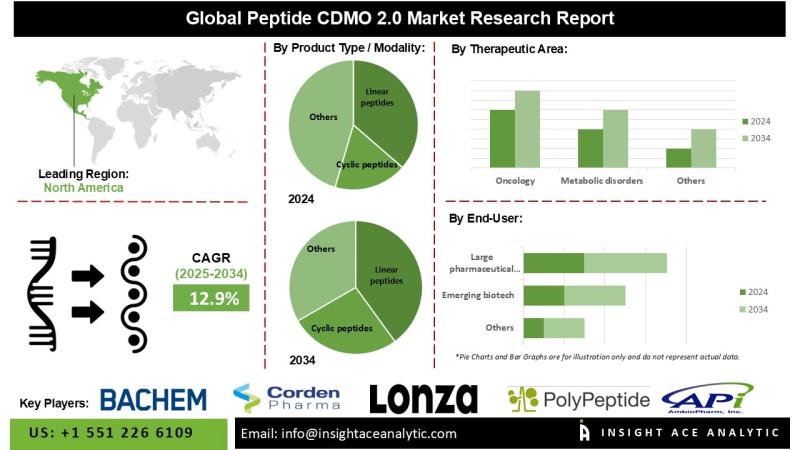
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Peptide CDMO 2.0 Market"-, By Product Type / Modality (Linear peptides, Cyclic peptides, Stapled peptides, Peptide-drug conjugates (PDCs), Peptide-oligonucleotide conjugates, GLP-1 and related long-acting analogues, Oral peptide formulations), By Scale of Operation (Preclinical, Clinical (Phase I-III), Commercial), By Business Model (Tech-Enabled CDMOs, Niche-Focused CDMOs, End-to-End Integrated CDMOs, Sustainability-Driven CDMOs), By Technology Platform (Solid Phase Peptide Synthesis (SPPS), Liquid Phase Peptide Synthesis (LPPS), Hybrid SPPS-LPPS, Enzymatic/biocatalytic synthesis, AI-assisted synthesis optimization), By Therapeutic Area (Oncology, Metabolic disorders, Infectious diseases, Rare & genetic disorders, Cardiovascular, Neurology), By End User (Large pharmaceutical companies, Emerging biotechs, Academic & research institutions), and Global Forecasts, 2025-2034 And Segment Revenue and Forecast To 2034."Global Peptide CDMO 2.0 Market Size is predicted to grow at a 12.9% CAGR during the forecast period for 2025-2034.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/3242
Peptide CDMOs 2.0 prioritize flexible and adaptable manufacturing platforms, including modular facility designs and multipurpose production systems, enabling rapid alignment with evolving regulatory requirements and diverse peptide therapeutic demands. The capacity to swiftly scale production-from accommodating seasonal fluctuations to large-scale manufacturing of high-demand drugs such as GLP-1 agonists-is critical for producing peptides with complex modifications and varied sequences.
By supplying research-grade peptides, developing peptide libraries for high-throughput screening, producing modified peptides (e.g., stapled, cyclic, or PEGylated) to enhance stability and activity, and supporting target validation studies to evaluate protein-protein interactions and receptor binding, peptide CDMOs are indispensable in early-stage drug discovery as well as commercial manufacturing.
The advent of Peptide CDMO 2.0 has redefined traditional manufacturing approaches through the integration of automation, AI-driven process optimization, continuous manufacturing, and advanced data analytics. These innovations have accelerated development timelines, improved yields, and enhanced cost efficiency. Scalability and reproducibility have been significantly improved by leveraging continuous-flow systems and advanced synthesis technologies, including solid-phase and liquid-phase peptide synthesis, enabling seamless transitions from clinical-scale production to full commercial manufacturing. In addition, these capabilities facilitate innovation in complex peptide formats, such as cyclic peptides and peptide-drug conjugates, while reducing time-to-market.
The growing demand for peptide therapeutics across therapeutic areas, including oncology, metabolic and cardiovascular disorders, and infectious diseases, is driving robust growth in the peptide CDMO market. Expansion of outsourcing strategies by both established pharmaceutical companies and emerging biotech firms, coupled with increasing regulatory acceptance of peptide-based drugs and rising investments in biologics research, further support market development. CDMOs equipped with advanced infrastructure, AI-enabled systems, and comprehensive end-to-end manufacturing capabilities are poised to play a pivotal role in accelerating the commercialization of next-generation peptide therapies, shaping the future of precision medicine.
List of Prominent Players in the Peptide CDMO 2.0 Market:
• Lonza Group AG
• CordenPharma
• Bachem Holding AG
• AmbioPharm
• PolyPeptide Group
• Evonik Health Care
• WuXi AppTec / WuXi TIDES
• Thermo Fisher Scientific (Patheon)
• Olon S.p.A.
• NOF Corporation
• Curapath
• eTheRNA Manufacturing
• Helix Biotech
• Phosphorex
• Creative Peptides
• Peptron Inc.
• Pepscan
• CSBio
• Neuland Laboratories
• Asymchem
• Sai Life Sciences
• AmbioPharm Shanghai
• Hybio Pharmaceutical
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2025-04
Market Dynamics
Drivers:
The rising global prevalence of chronic conditions, including obesity, cancer, and cardiovascular disorders, is driving significant demand for peptide and oligonucleotide therapeutics. These modalities offer personalized treatment tailored to individual patient profiles, making them particularly valuable for managing complex, long-term illnesses. As chronic disease rates continue to escalate worldwide, pharmaceutical companies are increasingly partnering with CDMOs to develop innovative peptide- and oligonucleotide-based therapies. The trend toward personalized medicine is further accelerating the demand for these therapeutics.
The growing adoption of peptides across therapeutic areas such as oncology, metabolic disorders (including diabetes and obesity), infectious diseases, neurology, and rare diseases is a key market driver. Blockbuster drugs, including GLP-1 receptor agonists such as semaglutide and liraglutide, underscore the need for scalable manufacturing solutions to meet surging global demand.
Additionally, modified peptides with enhanced stability, bioavailability, and efficacy-such as cyclic, stapled, PEGylated, and lipidated variants-are gaining traction. The rapid growth of Peptide-Drug Conjugates (PDCs) and Peptide-Oligonucleotide Conjugates further reflects the industry's shift toward precision and individualized medicine, highlighting the critical role of advanced CDMO capabilities.
Challenges:
High production costs and the technical complexity of peptide synthesis present significant challenges for startups and smaller firms. Regulatory frameworks governing peptide therapies are often stringent, requiring compliance with multiple standards set by authorities such as the U.S. Food and Drug Administration (FDA). Extended timelines and elevated costs associated with regulatory approvals may deter some companies from pursuing peptide-based therapeutics.
Regional Trends:
North America is projected to maintain the largest market share during the forecast period, driven by substantial investments in peptide therapeutics and a leadership position in biopharmaceutical research and development. Peptide CDMOs in the region are well-positioned to provide specialized synthesis and development services aligned with the growing emphasis on biologics and personalized medicine, including the production of high-purity linear and cyclic peptides.
Meanwhile, the Asia Pacific region is expected to register the fastest market growth, owing to favorable cost structures, expanding pharmaceutical infrastructure, and increasing demand for peptide therapeutics. CDMOs in this region are adopting advanced technologies such as AI-driven process optimization, continuous-flow synthesis, and automated solid-phase peptide synthesis (SPPS) to enhance efficiency and innovation. For instance, in January 2024, WuXi TIDES expanded its capacity for linear and cyclic peptides by establishing two state-of-the-art manufacturing facilities in Changzhou and Taixing, China, integrating automated SPPS and digitalized processes to support the growing therapeutic pipeline.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customization/3242
Recent Developments:
• In January 2024, WuXi AppTec launched two new peptide manufacturing plants, one in Changzhou and another at their new Taixing API site in China, tripling their overall peptide synthesis capacity and increasing total Solid-Phase Peptide Synthesis (SPPS) reactor volume to 32,000 liters. These advanced facilities use digital operations and automated solvent delivery systems to improve production efficiency, consistency, and scalability.
• In May 2023, PolyPeptide and Numaferm signed a Preferred Partner Collaboration Agreement for peptide development and production, utilizing Numaferm's biochemical production platform and sustainable peptide manufacturing expertise, as well as PolyPeptide's cGMP manufacturing capabilities, regulatory expertise, and market access. The company specializes in the development and production of peptides and proteins. The parties have committed to maintaining the confidentiality of the agreement's specifics.
Global Peptide CDMO 2.0 Market- By Product Type / Modality
• Linear peptides
• Cyclic peptides
• Stapled peptides
• Peptide-drug conjugates (PDCs)
• Peptide-oligonucleotide conjugates
• GLP-1 and related long-acting analogues
• Oral peptide formulations
Global Peptide CDMO 2.0 Market - By Scale of Operation
• Preclinical
• Clinical (Phase I-III)
• Commercial
Global Peptide CDMO 2.0 Market - By Business Model
• Tech-Enabled CDMOs (automation, AI, data integration)
• Niche-Focused CDMOs (rare diseases, complex peptides)
• End-to-End Integrated CDMOs
• Sustainability-Driven CDMOs
Global Peptide CDMO 2.0 Market- By Technology Platform
• Solid Phase Peptide Synthesis (SPPS)
• Liquid Phase Peptide Synthesis (LPPS)
• Hybrid SPPS-LPPS
• Enzymatic/biocatalytic synthesis
• AI-assisted synthesis optimization
Global Peptide CDMO 2.0 Market - By Therapeutic Area
• Oncology
• Metabolic disorders (incl. obesity/diabetes)
• Infectious diseases
• Rare & genetic disorders
• Cardiovascular
• Neurology
Global Peptide CDMO 2.0 Market - By End User
• Large pharmaceutical companies
• Emerging biotech
• Academic & research institutions
Global Peptide CDMO 2.0 Market - By Region
North America-
• The US
• Canada
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• Southeast Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Mexico
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of the Middle East and Africa
Why should buy this report:
To receive a comprehensive analysis of the prospects for the global Peptide CDMO 2.0 Market. To receive an industry overview and future trends of the global Peptide CDMO 2.0 Market
To analyze the Peptide CDMO 2.0 Market drivers and challenges
To get information on the Peptide CDMO 2.0 Market. size value (US$ Mn) forecast till 2034
Major Investments, Mergers & Acquisitions in the Cloud-Based and AI-Driven Eye Tracking Systems industry
Read Overview Report- https://www.insightaceanalytic.com/report/peptide-cdmo-2-0-market/3242
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: https://www.insightaceanalytic.com/
Tel : +1 607 400-7072
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Peptide CDMO 2.0 Market Forecast to 2034 with Focus on End-to-End Integrated and Tech-Enabled CDMOs here
News-ID: 4263765 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
Automotive Lead Acid Battery Market Strategic Growth Drivers and Outlook 2026 to …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Lead Acid Battery Market Size, Share & Trends Analysis Report By Product (SLI and Micro-Hybrid Batteries), Type (Flooded, Enhanced Flooded, and VRLA), Customer Segment (OEM and Aftermarket), End User (Passenger Car, Light Commercial Vehicles, Heavy Commercial Vehicles, Two-Wheeler, and Three-Wheeler), and Application (Hybrid Vehicles, Electric Vehicles, Light Motor Vehicles, and Heavy Motor Vehicles)- Market…
Automotive Interior Market Investment Opportunities and Forecast 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Interior Market- (By Component Type (Center Stack, Head-up Display, Instrument Cluster, Rear Sear Entertainment, Dome Module, Headliner, Seat, Interior Lighting Door Panel, Center Console, Adhesives & Tapes, Upholstery, Others), By Material (Leather, Fabric, Vinyl, Wood, Glass Fiber Composite, Carbon Fiber Composite, Metal), By Level of Autonomy (Semi-Autonomous, Autonomous, Non-Autonomous),By Electric Vehicle (Battery Electric Vehicle…
Artificial General Intelligence Market Future Landscape and Industry Evolution 2 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Artificial General Intelligence (AGI) Market - (By Type of Offering (Hardware, Software and Service), Type of Technology (Machine Learning, Deep Learning, Natural Language Processing and Robotics), Mode of Deployment (Cloud-based, On Premise and Web-based), Type of AI (Weak AI, Strong AI and Superintelligence), Type of Processing (Image, Text and Voice Processing), Company Size (SMEs and…
Allogenic Cell Therapies Market Revenue Trends and Growth Potential 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Allogenic Cell Therapies Market- by Cell Type(Cardiosphere-Derived Cells (CDCs), Fibroblasts, T-cells, Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs) and Others),Tissue Source(Skin, Blood, PBC, BM and Others), Indication (Acute graft-versus-host disease (GVHD), Chronic Ulcers and Diabetic Foot Ulcers, Osteoarthritis, Crohn's Disease, Cardiovascular Disease, Solid Tumors/Cancers and Others (Alzheimer's Disease, etc.)), Trends, Industry Competition Analysis, Revenue…
More Releases for Peptide
ShiLai Peptide to Invest $32 Million in State-of-the-Art Peptide Laboratory in H …
ShiLai Peptide's CEO, Luo Binhua, said the investment reflects the company's commitment to a "new science model" that integrates research-driven development, controlled production, and specialized customer support.
ShiLai Peptide [https://retatrutidesupplier.com/], a leading provider of high-purity, customizable research peptides, announced plans to invest $32 million to build a state-of-the-art peptide research and production laboratory in Hangzhou. The facility is designed to meet world-class GMP standards, aiming to strengthen ShiLai's global supply of…
Copper Peptide GHK-Cu Market: Empowering Beauty and Health Innovations with Adva …
The global copper peptide GHK-Cu market is poised for transformative growth as innovative cosmetic and pharmaceutical formulations increasingly incorporate these bioactive peptides to promote skin rejuvenation, wound healing, and overall wellness. Driven by technological advancements, growing consumer awareness of anti-aging solutions, and an expanding portfolio of product applications, the market is set to evolve rapidly in the coming years. This industry provides an in-depth analysis of market information, key growth…
Shaping the Cell Penetrating Peptide Market in 2025: Innovative Peptide Drug Dis …
How Big Is the Cell Penetrating Peptide Market Expected to Be, and What Will Its Growth Rate Be?
In recent times, the market size for cell penetrating peptides has expanded swiftly. The market is projected to rise from a value of $1.87 billion in 2024 to $2.16 billion in 2025, growing at a compound annual growth rate (CAGR) of 15.6%. The historic period's growth can be credited to an amplified comprehension…
Cancer Peptide Drugs Market
Global Peptide Cancer Drug Market Size, Dosage, Drug Price, Sales & Clinical Trials Insight 2030 Report Highlights:
• Global Peptide Cancer Drug Market Insight By Region & Indication
• Global Peptide Cancer Drug Market Opportunity: > US$ 18 Billion
• Approved Peptide Cancer Drugs: > 30 Drugs
• Approved Peptide Cancer Drugs Sales Insights, Patent, Dosage and Price Analysis
• Peptide Cancer Drugs Clinical Trials Insight By Company, Country, Indication and Phase
• Insight On Peptide Cancer Drugs In Clinical Trials: >…
Global Adjuvant Peptide Market Size,Share, Research and Forecast,2023-2028| Pept …
The global Adjuvant Peptide market is carefully researched in the report while largely concentrating on top players and their business tactics, geographical expansion, market segments, competitive landscape, manufacturing, and pricing and cost structures. Each section of the research study is specially prepared to explore key aspects of the global Adjuvant Peptide market. For instance, the market dynamics section digs deep into the drivers, restraints, trends, and opportunities of the global…
Peptide Modifications For PEGylation
PEGylation is the process of covalently attaching polyethylene glycol (PEG) polymer chains to peptides. By increasing their molecular mass and shielding them from proteolytic enzymes, PEGylation improves the pharmacokinetics of peptides and proteins. PEGylation reduces renal clearance and results in more sustained absorption after subcutaneous administration, as well as restricted distribution. PEGylations have been shown to significantly improve water solubility, biocompatibility, immunogenicity, and other physico-chemical properties. It is an established…