Press release
Viral Vector & Plasmid DNA Manufacturing Market to Reach USD 23.9 Billion by 2035, Growing at 15.7% CAGR | Transparency Market Research
The global viral vector & plasmid DNA manufacturing market is witnessing an extraordinary growth trajectory, reflecting the rapid advancement of gene and cell therapy technologies worldwide. Valued at US$ 4.8 billion in 2024, the market is projected to reach an impressive US$ 23.9 billion by 2035, expanding at a compound annual growth rate (CAGR) of 15.7% between 2025 and 2035. The surge is primarily driven by the increasing adoption of gene therapies for the treatment of rare and genetic disorders, technological advancements in production processes, and the growing number of clinical trials and therapy approvals.Explore pivotal insights and conclusions from our Report in this sample - https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=30428
Market Overview: Viral vectors and plasmid DNA play a foundational role in cell and gene therapy manufacturing and vaccine development. These biological tools are crucial for delivering therapeutic genes into human cells, offering potential cures for genetic and infectious diseases. As global healthcare pivots toward personalized and precision medicine, the demand for reliable, scalable, and GMP-compliant viral vector and plasmid DNA production continues to rise sharply.
According to market analysis, viral vectors accounted for 62.4% of total market share in 2024, establishing themselves as the dominant segment due to their efficiency and versatility in gene delivery. North America led the global market with a 43.8% share, driven by strong biotech infrastructure, favorable regulatory frameworks, and significant private and public investments.
Key Drivers of Market Growth
1. Expanding Gene Therapy Pipeline
The continuous expansion of the gene therapy pipeline remains the strongest catalyst for market growth. As biopharmaceutical companies advance innovative gene-based therapies, the need for high-quality viral vectors and plasmid DNA intensifies. Each novel therapy requires unique vector constructs, compelling manufacturers to enhance production scalability, process standardization, and GMP compliance.
2. Rise in Clinical Trials and Therapy Approvals
A notable increase in clinical trials and regulatory approvals for gene and cell therapy products has significantly boosted the need for large-scale manufacturing of viral vectors and plasmid DNA. As therapies progress through late-stage clinical trials, manufacturers are prioritizing automation, process optimization, and robust quality systems to meet stringent global regulatory standards.
3. Technological Innovations
Innovations such as single-use bioreactors, perfusion processes, and closed-system workflows are revolutionizing the bioprocessing landscape. These technologies enhance manufacturing flexibility, minimize contamination risks, and reduce turnaround time. Moreover, advancements in downstream purification, such as high-capacity chromatography and nuclease clearance, are improving product yield and purity.
4. Strategic Collaborations and CDMO Partnerships
The growing collaboration between contract development and manufacturing organizations (CDMOs) and biopharmaceutical companies is propelling the industry. These partnerships enable end-to-end manufacturing solutions, from plasmid supply to fill-finish operations, optimizing time-to-market and ensuring consistent quality.
Latest Market Trends
The viral vector & plasmid DNA manufacturing market is undergoing a transformation driven by flexible manufacturing models, sustainability goals, and digital automation:
• Adoption of Single-Use Technologies: Companies are shifting toward single-use bioreactor systems to enable faster product changeover, minimize cross-contamination, and reduce operational costs.
• Automation and AI Integration: Smart manufacturing and data-driven process analytics are improving scalability, reducing human error, and ensuring consistent quality in vector production.
• Sustainability Initiatives: Market leaders are emphasizing energy-efficient production and waste reduction strategies, aligning with global sustainability objectives.
• Regionalization of Supply Chains: To ensure supply security and rapid response capabilities, companies are investing in modular facilities and multi-site manufacturing hubs across key global markets.
Key Players and Industry Leaders
The market features a highly competitive landscape dominated by leading biotech and pharmaceutical companies focusing on capacity expansion, innovation, and regulatory excellence. Prominent players include:
• Thermo Fisher Scientific Inc.
• Lonza Group AG
• Merck KGaA
• Takara Bio Inc.
• Sanofi
• Aldevron LLC
• FUJIFILM Biotechnologies
• WuXi Biologics
• REGENXBIO Inc.
• AGC Biologics
• Batavia Biosciences B.V.
• Hillgene Biopharma Co., Ltd.
• Revvity
• Wacker Chemie AG
These companies are heavily investing in automation, capacity building, and modular production systems to meet rising global demand. Their strategies include process standardization, regulatory alignment, and capacity reservation models to optimize scalability and compliance.
Recent Developments
• June 2025 - ProBio Expansion:
ProBio inaugurated its Cell and Gene Therapy Center of Excellence at Princeton West Innovation Campus in New Jersey. The 128,000 sq. ft. GMP facility focuses on high-quality plasmid DNA and viral vector manufacturing, strengthening the company's position in the AAV and lentiviral vector platforms.
• June 2025 - ArcticZymes Technologies ASA:
ArcticZymes expanded its GMP product line by launching M-SAN HQ GMP, a high-performance nuclease designed for virus vector manufacturing. This innovation provides a regulatory-compliant solution for host cell DNA elimination, enhancing viral vector purity and safety.
These developments underscore the industry's focus on innovation, compliance, and scalable production capacity, all crucial for accelerating gene therapy commercialization.
Buy this Premium Research Report for a deep dive into essential data - https://www.transparencymarketresearch.com/checkout.php?rep_id=30428<ype=S
Market Opportunities and Challenges
Opportunities
• Growing Focus on Personalized Medicine:
The rise in precision and patient-specific therapies presents new opportunities for customized viral vector and plasmid DNA production platforms.
• Emerging Markets:
Asia Pacific and Latin America offer untapped potential due to increasing biotech investments and supportive government initiatives.
• Technological Convergence:
Integration of AI-driven process control and continuous biomanufacturing could drastically improve production efficiency.
Challenges
• High Manufacturing Costs:
The production of viral vectors and plasmid DNA involves complex, capital-intensive processes with strict quality standards, leading to high costs.
• Regulatory Complexities:
Varying regulatory frameworks across regions create challenges for harmonizing production and quality assurance.
• Skilled Workforce Shortage:
The industry faces a talent gap in specialized bioprocessing and GMP operations, slowing down capacity expansion in emerging markets.
Future Outlook
The future of the viral vector & plasmid DNA manufacturing market is characterized by innovation, flexibility, and integration. With the continued success of gene therapy trials and the introduction of next-generation vector technologies, the market is poised for exponential growth.
Experts predict that by 2035:
• Viral vector manufacturing will become increasingly automated and modular, supporting rapid scalability.
• End-to-end CDMO solutions will dominate the supply chain, reducing time-to-market.
• Regional production hubs in Asia and Europe will play a vital role in global supply diversification.
• The use of artificial intelligence (AI) and machine learning (ML) will revolutionize process optimization, predictive maintenance, and quality control.
Overall, the market's growth trajectory reflects the global momentum toward advanced genetic medicine, transforming the landscape of modern healthcare.
Market Segmentation
The viral vector & plasmid DNA manufacturing market is segmented by vector type, workflow, application, scale, end-user, and region:
By Vector Type:
• Viral Vectors (Adenovirus, Lentivirus, AAV, Retrovirus, Others)
• Plasmid DNA
By Workflow:
• Upstream (Vector Amplification, Editing & Expansion)
• Downstream (Purification, Fill-Finish)
By Application:
• Cell and Gene Therapies
• Vaccine Development
• Antisense & RNAi Therapies
• Others (Stem Cell Research, etc.)
By Scale:
• Preclinical
• Clinical
• Commercial
By End-User:
• Pharmaceutical & Biotechnology Companies
• Contract Manufacturing Organizations (CMOs)
• Academic & Research Institutions
By Region:
• North America (U.S., Canada)
• Europe (Germany, U.K., France, Italy, Spain, Netherlands)
• Asia Pacific (China, India, Japan, South Korea, ASEAN, Australia)
• Latin America (Brazil, Mexico, Argentina)
• Middle East & Africa (GCC, South Africa)
Why Buy This Report?
This comprehensive market report provides a strategic roadmap for stakeholders seeking to capitalize on the booming viral vector and plasmid DNA manufacturing industry. Key benefits include:
• In-depth Market Analysis: Covers quantitative forecasts (2025-2035), segment insights, and value chain analysis.
• Competitive Landscape: Detailed profiles of top global manufacturers and emerging players.
• Regional Insights: Comparative assessment of growth opportunities across major markets.
• Technology Outlook: Evaluation of the latest bioprocessing innovations and automation trends.
• Strategic Recommendations: Actionable insights for investors, manufacturers, and policymakers to strengthen market position and drive long-term growth.
Explore Latest Research Reports by Transparency Market Research:
PRP and PRF in Cosmetics Market: https://www.transparencymarketresearch.com/prp-and-prf-in-cosmetics-market.html
Genome Engineering Market: https://www.transparencymarketresearch.com/genome-editing-engineering-market.html
Genome Editing Market: https://www.transparencymarketresearch.com/genome-editing-market-report.html
Multiplex PCR Market: https://www.transparencymarketresearch.com/multiplex-pcr-market.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact Us:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Viral Vector & Plasmid DNA Manufacturing Market to Reach USD 23.9 Billion by 2035, Growing at 15.7% CAGR | Transparency Market Research here
News-ID: 4259884 • Views: …
More Releases from Transparency Market Research
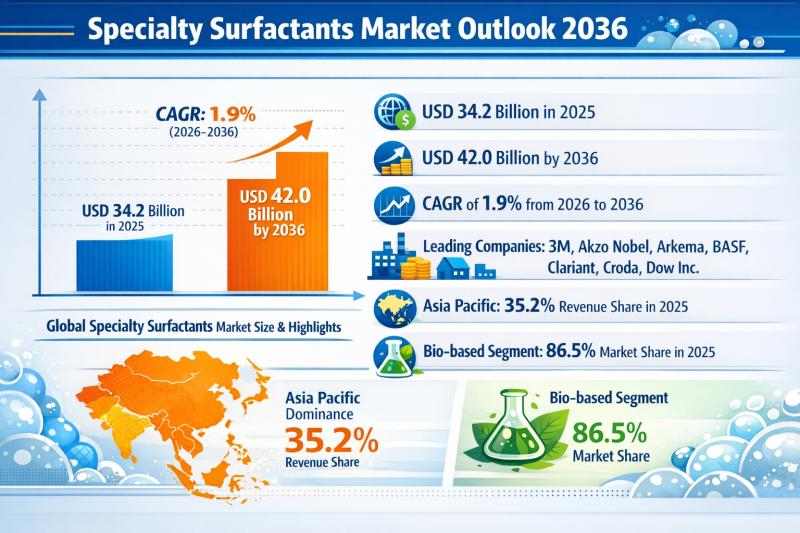
Specialty Surfactants Market Outlook 2036: Bio-Based Dominance, 1.9% CAGR Growth …
The global Specialty Surfactants Market reached a valuation of US$ 34.2 Billion in 2025 and is projected to climb to US$ 42.0 Billion by 2036, expanding at a steady CAGR of 1.9% from 2026 to 2036. While the growth rate reflects a mature yet stable industry, the sector continues to witness structural shifts driven by sustainability mandates, innovation in bio-based formulations, and expanding applications across end-use industries.
Asia Pacific accounted for…
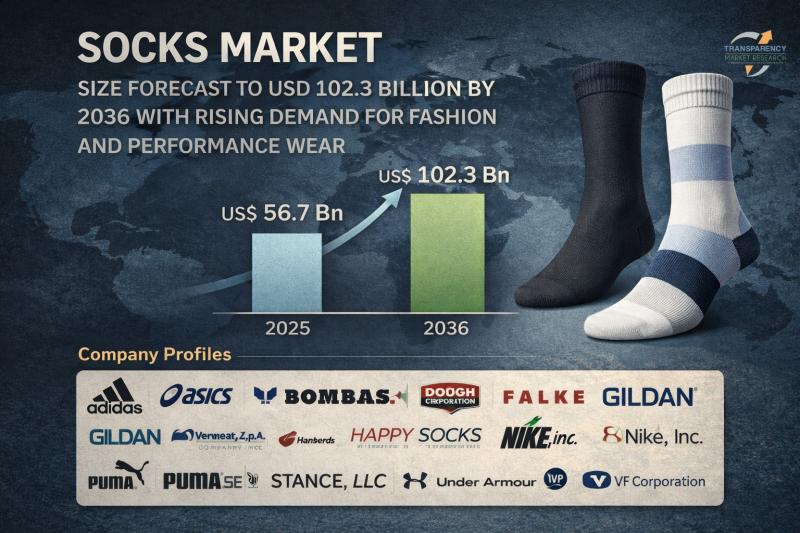
Socks Market Size Forecast to USD 102.3 Billion by 2036 with Rising Demand for F …
Socks Market Outlook 2036
The global socks market was valued at US$ 56.7 Bn in 2025 and is projected to reach US$ 102.3 Bn by 2036, expanding at a steady CAGR of 5.5% from 2026 to 2036. Market growth is driven by increasing demand for comfortable and functional apparel, rising fashion consciousness, expanding sports participation, and the rapid growth of e-commerce platforms.
👉 Get your sample market research report copy today@ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=9470
Market…
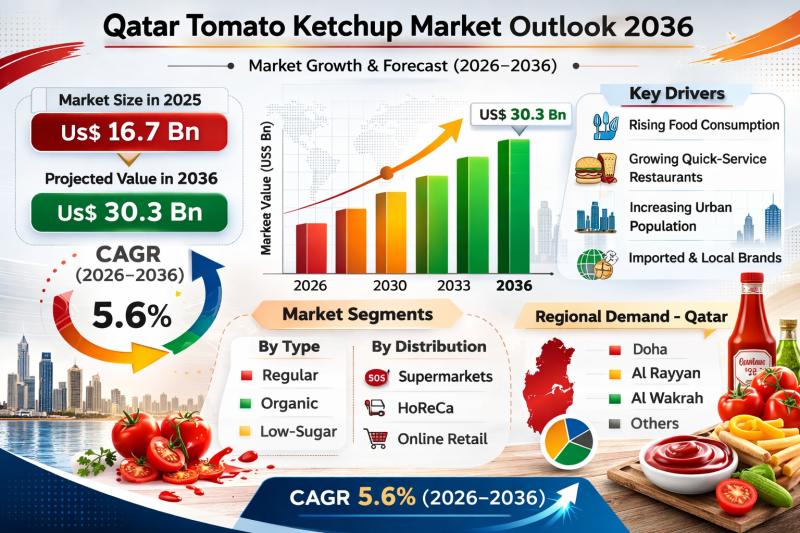
Qatar Tomato Ketchup Market Expanding at 5.6% CAGR Through 2036 - By Type / By P …
The Qatar Tomato Ketchup Market was valued at US$ 16.7 Bn in 2025 and is projected to reach US$ 30.3 Bn by 2036, expanding at a CAGR of 5.6% from 2026 to 2036. The steady growth trajectory reflects increasing consumption of convenience foods, rising demand from quick-service restaurants (QSRs), and continued innovation in product variants and packaging formats.
Get a concise overview of key insights from our Report in this sample…
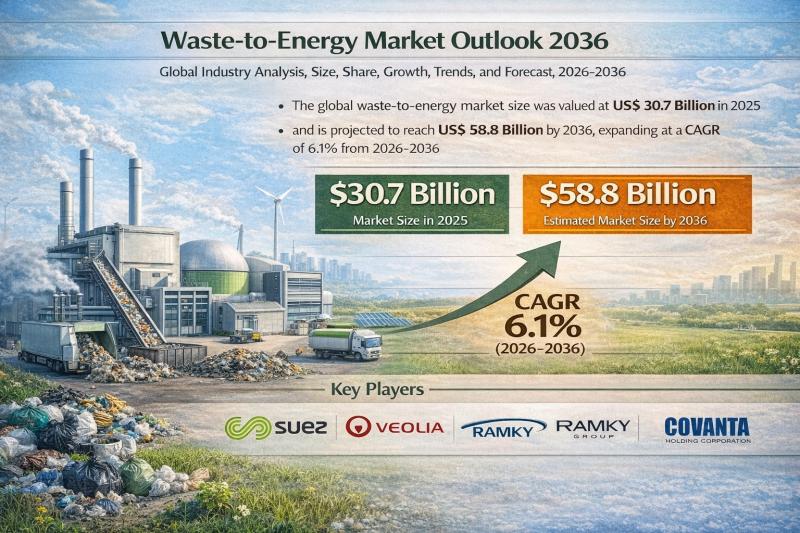
Waste-to-Energy Market to Reach US$ 58.8 Billion by 2036 at 6.1% CAGR | Transpar …
The global waste-to-energy (WtE) market is entering a dynamic growth phase as governments and industries intensify efforts to transition toward sustainable waste management and renewable power generation. Waste-to-energy refers to the process of converting municipal solid waste (MSW), agricultural waste, and other refuse into usable forms of energy such as electricity, heat, and fuel through technologies including incineration, gasification, pyrolysis, and anaerobic digestion.
As environmental pressures mount and landfill capacity shrinks,…
More Releases for DNA
High-Quality Plasmid DNA Fuels Growth in Global DNA Plasmid Manufacturing Market
🌍 Market Overview
The DNA Plasmid Manufacturing Market is experiencing robust growth as advancements in cell & gene therapy, DNA vaccines, and genetic engineering continue to expand globally. Plasmid DNA plays a critical role as a raw material in the development of advanced therapies, fueling demand across biopharmaceutical research and production.
Key factors driving the market include:
Increasing adoption of gene and cell therapies
Rising prevalence of chronic and rare genetic disorders
Expansion of DNA-based…
DNA Synthesis Market Increasing Demand for Synthetic Genes and DNA Sequences
As demonstrated by Precision Business Insights (PBI), the latest report, the global DNA synthesis market was valued at USD 3,702.0 million in 2023 and is expected to reach USD 10,289.5 million by 2029, growing at a CAGR of 18.6% during the forecast period 2024-2030. The key drivers for the growth of the global DNA synthesis market include increasing demand for synthetic genes and DNA sequences, growing applications in the agriculture…
Wealth DNA Code Review Legit Price? (Wealth Manifestation DNA Code Audio Frequen …
Wealth DNA Code Wealth DNA Code is a digital program with seven minutes of soundtracks that manifest and listen to daily to activate the "Wealth DNA," which is part of your DNA to help you attract wealth by making money a part of your mentality and making your dreams to come true.
https://bit.ly/Visit-The-Official-Website-Here-To-Order-Wealth-DNA-Code
Making money, creating assets as well as increasing wealth are the primary objectives that every human being has to…
DNA Paternity Testing Market Size [2022-2029] -DNA Diagnostics Center, EasyDNA, …
A recent market research report added to repository of MR Accuracy Reports is an in-depth analysis of global DNA Paternity Testing. On the basis of historic growth analysis and current scenario of DNA Paternity Testing place, the report intends to offer actionable insights on global market growth projections. Authenticated data presented in report is based on findings of extensive primary and secondary research. Insights drawn from data serve as excellent…
DNA Paternity Testing Market Trends 2020 | Growth by Top Companies: DNA Diagnost …
The report begins with the overview of the DNA Paternity Testing Market and offers throughout development. It presents a comprehensive analysis of all the regional and major player segments that gives closer insights upon present market conditions and future market opportunities along with drivers, trending segments, consumer behaviour, pricing factors and market performance and estimation. The forecast market information, SWOT analysis, DNA Paternity Testing market scenario, and feasibility study are…
DNA Paternity Testing Market Rapidly Growing in Healthcare, Competitor Analysis …
The exclusive research report on the Global DNA Paternity Testing Market 2020 examines the market in detail along with focusing on significant market dynamics for the key players operating in the market. Global DNA Paternity Testing Industry research report offers granulated yet in-depth analysis of revenue share, market segments, revenue estimates and various regions across the globe.
Overview of Global DNA Paternity Testing Market:
This report studies the Global DNA Paternity Testing…
