Press release
Bioprocess Validation Market to Reach USD 1.2 Billion by 2035, Driven by Surge in Biopharmaceutical Demand and Stringent Regulatory Standards | TMR
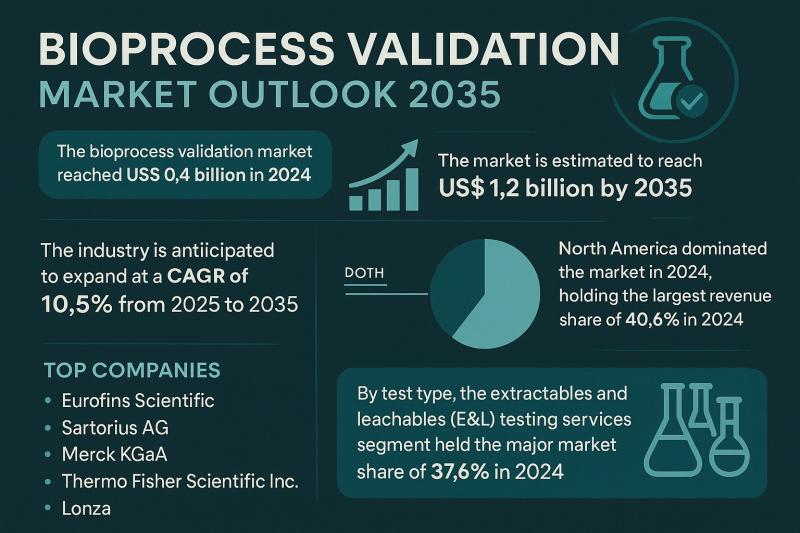
The global Bioprocess Validation Market is projected to experience exceptional growth over the next decade, expanding from US$ 0.4 billion in 2024 to over US$ 1.2 billion by 2035, registering a strong compound annual growth rate (CAGR) of 10.5% between 2025 and 2035. This upward trajectory underscores the increasing reliance of the global pharmaceutical and biotechnology industries on robust validation frameworks to ensure the safety, efficacy, and consistency of biopharmaceutical products.Preview crucial insights and findings from our Report in this sample -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=72540
This growth is primarily driven by the widespread adoption of biologics, biosimilars, and advanced therapies, alongside the tightening of global regulatory standards by agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The evolution of validation practices through the integration of automation, artificial intelligence, and advanced analytical tools is also enhancing process reliability, reducing operational risks, and fostering a more sustainable and compliant biomanufacturing ecosystem.
Key Market Highlights
The global bioprocess validation market demonstrated notable performance in 2024, reaching US$ 0.4 billion, and is expected to triple its valuation by 2035. This remarkable growth will be supported by advancements in validation technologies, the rapid scaling of biomanufacturing capacities, and the continuous demand for biopharmaceuticals addressing unmet medical needs.
North America continues to hold a commanding position, accounting for 40.6% of the total market share in 2024. The region's dominance is underpinned by a well-established regulatory environment, a strong concentration of biopharmaceutical manufacturers, and substantial investments in R&D infrastructure.
Among service segments, Extractables and Leachables (E&L) Testing emerged as the largest contributor, representing 37.6% of the global market share in 2024. This is attributed to the rising deployment of single-use systems and the increasing scrutiny of potential contaminants in drug products.
The market's leading companies - including Eurofins Scientific, Sartorius AG, Merck KGaA, Thermo Fisher Scientific Inc., and Lonza - continue to invest in expanding analytical capabilities, developing high-throughput validation tools, and strengthening their global service networks to meet the evolving demands of pharmaceutical and biotechnology clients.
Market Overview
The Bioprocess Validation Market plays a critical role in ensuring that biopharmaceutical manufacturing processes consistently yield products meeting stringent quality and regulatory requirements. As biopharmaceutical products become more complex and globally distributed, the need for comprehensive validation of equipment, analytical methods, and production environments has intensified.
The growing demand for therapeutic proteins, vaccines, and biosimilars has propelled manufacturers to adopt advanced validation methodologies to maintain compliance and mitigate risks. Simultaneously, the industry is witnessing a paradigm shift toward outsourced validation services, allowing pharmaceutical and biotechnology companies to leverage specialized expertise and state-of-the-art facilities offered by third-party validation partners.
Technological innovations, particularly in automation, process analytics, and data management, are redefining validation efficiency by minimizing human error, improving traceability, and ensuring continuous quality monitoring. Furthermore, the rising emphasis on personalized and precision medicine is creating new opportunities for specialized validation protocols that can accommodate small-batch, high-variability production environments.
Key Developments
The bioprocess validation sector continues to evolve rapidly through strategic collaborations, technological advancements, and facility expansions.
In July 2025, Dante Omics AI unveiled its GPU-accelerated multi-omics platform designed to integrate genomics, metabolomics, and transcriptomics data. This innovation marks a milestone in molecular-level validation, enabling real-time analysis and driving progress in personalized medicine and disease modeling.
In May 2025, Pluto Biosciences secured US$ 3.6 million in funding to expand its AI-driven multi-omics data platform. This solution allows pharmaceutical firms to execute bioinformatics pipelines and visualize datasets without coding, accelerating the adoption of computational biology in regulatory validation and clinical research.
Furthermore, WuXi Biologics achieved European Medicines Agency (EMA) approval for its state-of-the-art commercial production facility in Dundalk, Ireland - a pivotal step that strengthens Europe's manufacturing and validation infrastructure for biologics.
These developments collectively highlight the sector's commitment to digital transformation, regulatory compliance, and enhanced process transparency across the biopharmaceutical value chain.
Discover key insights by visiting our in-depth report -
https://www.transparencymarketresearch.com/bioprocess-validation-market.html
Leading Market Players
The global bioprocess validation market is characterized by the presence of several key players that have established strong global footprints through diversified service portfolios, technological expertise, and strategic alliances.
Prominent participants include Eurofins Scientific, Sartorius AG, Merck KGaA, Thermo Fisher Scientific Inc., Lonza, Danaher Corporation, Charles River Laboratories, SGS Société Générale de Surveillance, LabCorp, Pall Corporation, Almac Group, METTLER TOLEDO, Cytiva, and Bio-Rad Laboratories, Inc.
These companies are focusing on continuous innovation through next-generation validation technologies, regulatory-aligned testing protocols, and data-integrated quality systems. Partnerships with biotechnology startups, the incorporation of artificial intelligence into validation analytics, and facility expansions across emerging markets are key strategies enabling them to strengthen their competitive advantage and capture emerging demand in Asia-Pacific and Europe.
Regional Insights
North America remains the global leader in the bioprocess validation market, driven by a mature biopharmaceutical sector, advanced manufacturing facilities, and robust regulatory oversight. The region benefits from significant government and private investments in expanding biomanufacturing capabilities. For instance, AstraZeneca's US$ 4.5 billion manufacturing expansion in Virginia aligns with its broader US$ 50 billion investment plan aimed at bolstering domestic production and research infrastructure by 2030.
Europe also plays a vital role in global market development, with countries such as Germany, the U.K., and France leading in regulatory compliance and validation adoption. Europe's ongoing focus on biosimilars and advanced therapy medicinal products (ATMPs) continues to drive validation service requirements across the continent.
Meanwhile, the Asia Pacific region is poised for the fastest growth during the forecast period, supported by expanding CMO and CDMO networks, rising government support for biopharmaceutical manufacturing, and the region's increasing role in global clinical trials. Nations such as China, India, South Korea, and Japan are heavily investing in capacity expansion and quality assurance technologies to align with international validation standards.
Buy this Premium Research Report for a deep dive into essential data -
https://www.transparencymarketresearch.com/checkout.php?rep_id=72540<ype=S
Analyst's Perspective
Industry experts emphasize that bioprocess validation is no longer a regulatory obligation but a strategic imperative for ensuring quality, consistency, and market competitiveness in biopharmaceutical production. As the demand for complex biologics and cell- and gene-based therapies escalates, validation systems must evolve to incorporate AI-driven analytics, digital twins, and predictive modeling to manage risks and ensure traceability across the manufacturing lifecycle.
Analysts also highlight that companies integrating automated validation workflows and cloud-based data management systems are likely to experience significant cost advantages, faster time-to-market, and improved compliance with global quality frameworks. The bioprocess validation industry, therefore, stands at the intersection of technology, regulation, and innovation, defining the future of high-quality biomanufacturing worldwide.
Explore Latest Research Reports by Transparency Market Research:
Bioprocess Validation Market - https://www.transparencymarketresearch.com/bioprocess-validation-market.html
Viral Vectors & Plasmid DNA Manufacturing Market - https://www.transparencymarketresearch.com/viral-vectors-plasmid-dna-manufacturing-market.html
Multiomics Market - https://www.transparencymarketresearch.com/multiomics-market.html
Animal-Model Market - https://www.transparencymarketresearch.com/animal-model-market.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
Follow Us: LinkedIn| Twitter| Blog | YouTube
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Bioprocess Validation Market to Reach USD 1.2 Billion by 2035, Driven by Surge in Biopharmaceutical Demand and Stringent Regulatory Standards | TMR here
News-ID: 4259868 • Views: …
More Releases from Transparency Market Research
Gas Compressor Market Outlook 2036: Global Industry Expected to Reach US$ 41.0 B …
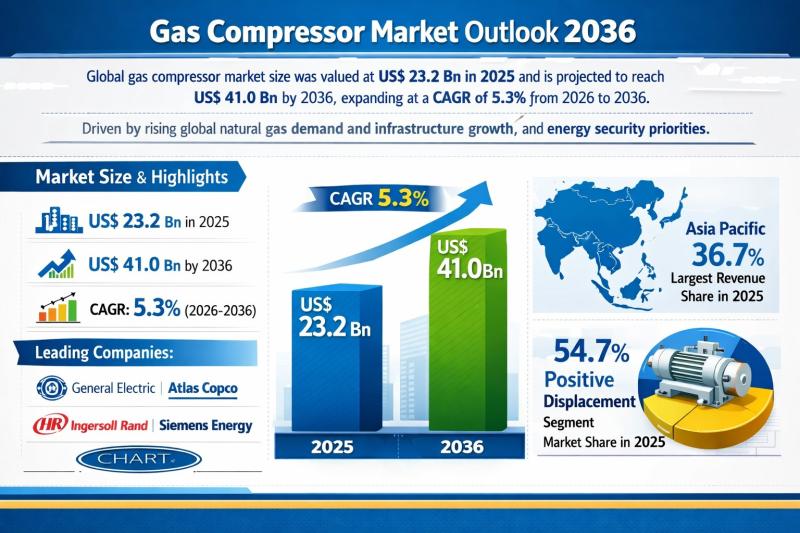
The global gas compressor market was valued at US$ 23.2 Bn in 2025 and is projected to reach US$ 41.0 Bn by 2036, expanding at a compound annual growth rate (CAGR) of 5.3% from 2026 to 2036. This steady growth trajectory reflects the structural importance of gas compression systems across upstream, midstream, and downstream gas value chains. Rising natural gas consumption, expansion of pipeline and LNG infrastructure, and national energy…
Anesthesia Drugs Market to be Worth USD 12.6 Bn by 2036 - By Drug / By Applicati …
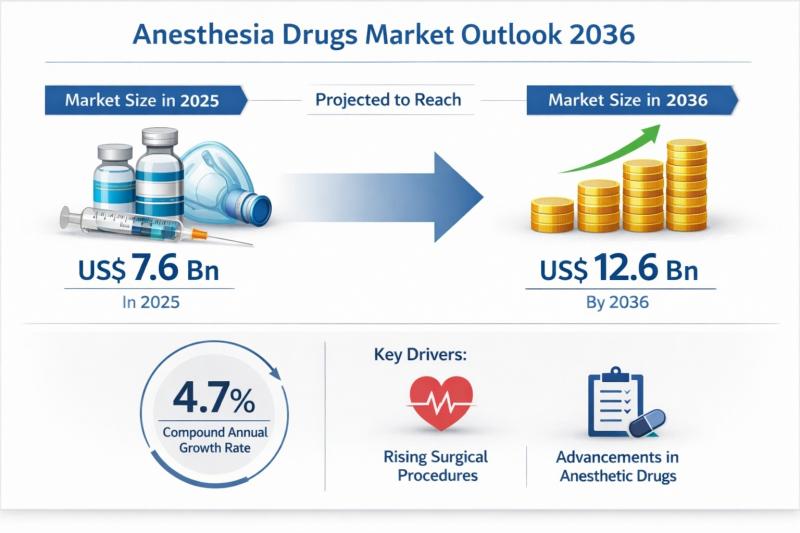
The global anesthesia drugs market was valued at US$ 7.6 billion in 2025 and is projected to reach US$ 12.6 billion by 2036, expanding at a compound annual growth rate (CAGR) of 4.7% from 2026 to 2036. This steady growth trajectory reflects the essential and non-substitutable role of anesthesia drugs in modern healthcare systems. As surgical interventions continue to rise globally-across both elective and emergency procedures-the demand for safe, effective,…
Single-Atom Catalysts Market Size is Expected to Expand from US$ 177.8 Million t …
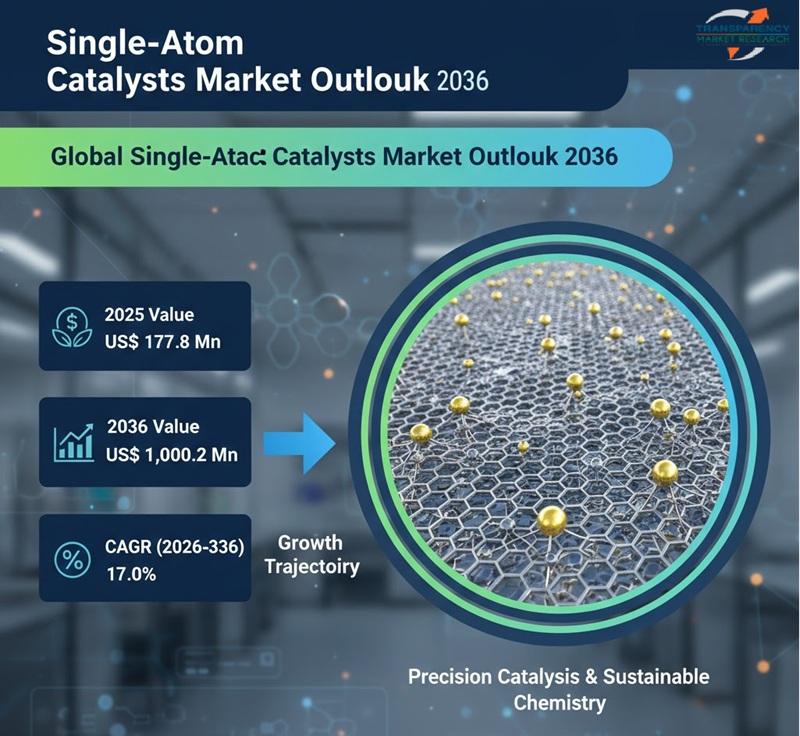
The global single-atom catalysts (SACs) market is poised for remarkable growth as industries seek highly efficient, cost-effective, and sustainable catalytic solutions. Valued at US$ 177.8 million in 2025, the market is projected to reach US$ 1,000.2 million by 2036, expanding at a robust compound annual growth rate (CAGR) of 17.0% from 2026 to 2036. This rapid expansion reflects the growing importance of advanced catalysis in energy, chemicals, environmental protection, and…
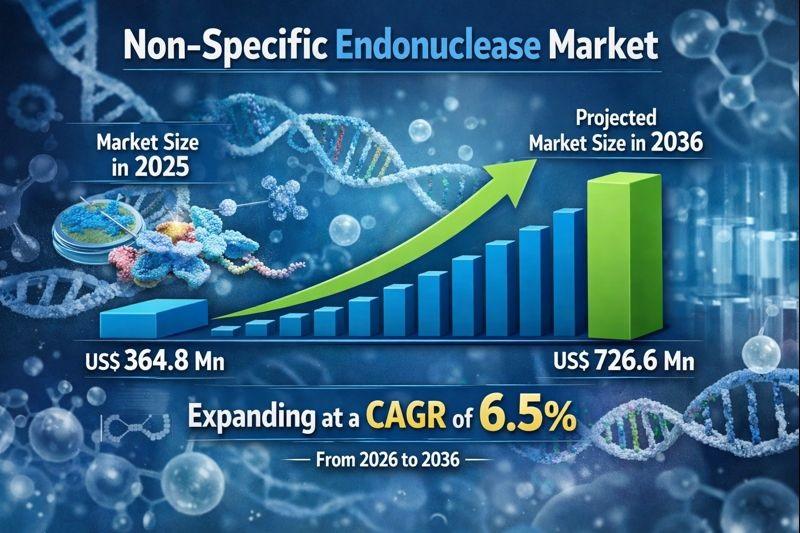
Non-specific Endonuclease Market to Reach USD 726.6 Million by 2036, Supported b …
The non-specific endonuclease market is witnessing steady growth, driven by the expanding use of molecular biology tools across biotechnology, pharmaceuticals, diagnostics, and academic research. Non-specific endonucleases are enzymes that cleave nucleic acids without requiring a specific recognition sequence, making them highly valuable for applications such as DNA/RNA degradation, sample preparation, viscosity reduction, and contamination control. Their broad activity profile differentiates them from restriction enzymes and enables versatile usage across multiple…
More Releases for Bioprocess
Bioprocess Automation Market Massive Growth opportunity Ahead
Introduction
Bioprocessing lies at the core of modern biotechnology, enabling the production of life-saving drugs, vaccines, and biologics. As demand for biologics and personalized medicines grows, the need for automation in bioprocessing has become increasingly critical. Bioprocess automation systems streamline manufacturing workflows, reduce variability, ensure compliance with stringent regulations, and enhance scalability-all while reducing costs and labor dependency.
The global Bioprocess Automation Market is on a steady growth trajectory, supported by rising…
Prominent Bioprocess Bags Market Trend for 2025: Product Innovations Transformin …
What industry-specific factors are fueling the growth of the bioprocess bags market?
The upward trend of biotechnology companies is set to stimulate the expansion of the bioprocess bag market. These companies utilize biological expertise and instruments to generate products and services for different industries. Bioprocess bags offer several advantages to the biotechnology industry such as minimized environmental harm, adaptability, cost-efficiency, swiftness, and prevention of contamination. For instance, the World Bank reported…
Prominent Bioprocess Bags Market Trend for 2025: Product Innovations Transformin …
What industry-specific factors are fueling the growth of the bioprocess bags market?
The upward trend of biotechnology companies is set to stimulate the expansion of the bioprocess bag market. These companies utilize biological expertise and instruments to generate products and services for different industries. Bioprocess bags offer several advantages to the biotechnology industry such as minimized environmental harm, adaptability, cost-efficiency, swiftness, and prevention of contamination. For instance, the World Bank reported…
Bioprocess Analyzers Market Report 2024 - Bioprocess Analyzers Market Demand And …
"The Business Research Company recently released a comprehensive report on the Global Bioprocess Analyzers Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
According to The Business Research Company's, The bioprocess analyzers market size…
Bioprocess Containers Market: Revolutionizing Biopharmaceutical Manufacturing
Explore the transformative impact of bioprocess containers in the biopharmaceutical industry, facilitating the production of biologics and biosimilars with greater efficiency and cost-effectiveness. With a projected market value of USD 9.3 billion by 2033, discover the drivers, trends, and innovations shaping the global bioprocess containers market at a remarkable CAGR of 11.1%.
Download Sample Copy with Graphs & List of Figures@
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=75155
Harnessing Single-Use Technologies: Driving Market Expansion
Delve into the rise in…
Bioprocess Containers Set to Achieve US$ 9.8 Billion till 2033 by Witnessing 2D …
The Global 𝐁𝐢𝐨𝐩𝐫𝐨𝐜𝐞𝐬𝐬 𝐂𝐨𝐧𝐭𝐚𝐢𝐧𝐞𝐫𝐬 Market is estimated to attain a valuation of US$ 9.8 Billion by the end of 2033, states a study by Transparency Market Research (TMR). Besides, the report notes that the market is prognosticated to expand at a CAGR of 11.8% during the forecast period, 2023 - 2033.
The key objective of the TMR report is to offer a complete assessment of the global market including major leading…