Press release
GMP-Grade IVT Enzymes for Therapeutic RNA Market Boosted by Advances in Enzyme Engineering for Improved Yield and RNA Stability
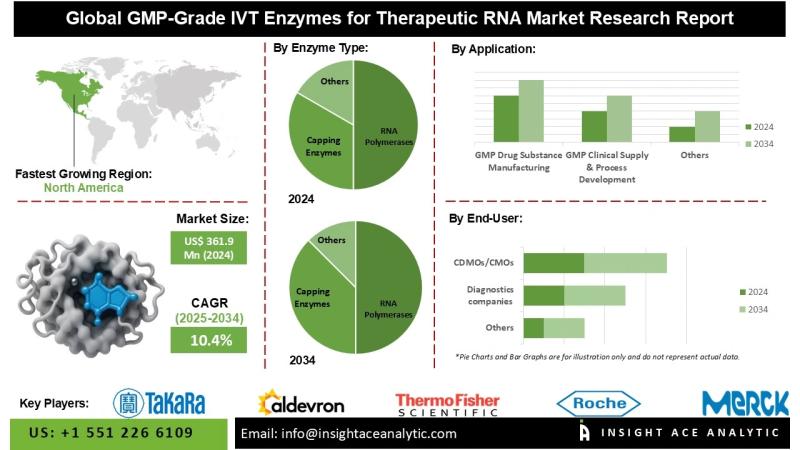
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "GMP-Grade IVT Enzymes for Therapeutic RNA Market"- By Enzyme Type (IVT Workflow)- (RNA Polymerases, Capping Enzymes, Tailing Enzymes - Poly(A) Polymerase, Template Generation Enzymes, Cleanup / Yield / Protection, Circular RNA Enzymes), By RNA Modality (mRNA, saRNA/replicons, circRNA, gRNAs/crRNAs (CRISPR), Other therapeutic RNAs), By Application / Use Stage (GMP Drug Substance Manufacturing, GMP Clinical Supply & Process Development (Phase I-III), Diagnostics/IVD, Translational Research with GMP continuity), By End User (Biopharma/Biotech sponsors (in-house manufacturing), CDMOs/CMOs, Diagnostics companies, Academic/Government Translational Centers), and Global Forecasts, 2025-2034 And Segment Revenue and Forecast To 2034."Global GMP-Grade IVT Enzymes for Therapeutic RNA Market Size is valued at USD 361.9 Mn in 2024 and is predicted to reach USD 923.0 Mn by the year 2034 at a 10.4% CAGR during the forecast period for 2025-2034.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/3248
Good Manufacturing Practice (GMP)-compliant, high-purity IVT (in vitro transcription) enzymes for therapeutic RNA are produced in accordance with GMP guidelines to facilitate the commercial, clinical, and large-scale synthesis of mRNA, saRNA, and other therapeutic RNAs. These enzymes are essential to the IVT process, which is the main way that RNA molecules are synthesized for use in gene editing, protein replacement therapy, cancer immunotherapies, and mRNA vaccines. The most commonly employed enzyme in therapeutic applications is T7 polymerase, while other important enzymes include RNA polymerases like T7, T3, and SP6, which catalyze RNA synthesis from a DNA template.
RNA stability, translation efficiency, and immune evasion all depend on the addition of a functional 5' cap, which is ensured by capping enzymes like vaccinia capping enzyme or co-transcriptional analogs like CleanCap. To improve stability and translation, poly(A) polymerase adds a poly(A) tail to the 3' end of the RNA, and RNase inhibitors shield transcripts from deterioration during synthesis. Lastly, after transcription, GMP-grade DNase I is used to eliminate the DNA template, ensuring the creation of clean, high-quality RNA suitable for clinical use.
The GMP drug substance manufacturing relies on RNase inhibitors, poly(A) polymerase, vaccinia capping enzyme, and IVT enzymes like T7 RNA polymerase to facilitate the synthesis of therapeutic RNA. The transcription of RNA from DNA templates, the addition of 5′ caps and 3′ poly(A) tails, and the maintenance of RNA stability during large-scale commercial production are all crucial tasks carried out by these enzymes. GMP-grade IVT enzymes are becoming more and more in demand as the need for high-purity mRNA to enable gene treatments, vaccines, and other RNA-based pharmaceuticals increases.
Enzyme engineering advancements are assisting in the resolution of immunogenicity and process efficiency issues, hence solidifying their position as essential instruments in commercial manufacturing. Advances in technologies like microfluidics and continuous IVT processes are further improving scalability and cost-effectiveness, which is driving a greater reliance on GMP-grade enzymes optimized for commercial production. The mRNA vaccine segment in particular has emerged as a major driver of enzyme demand.
List of Prominent Players in the GMP-Grade IVT Enzymes for Therapeutic RNA Market:
• New England Biolabs (NEB)
• Thermo Fisher Scientific
• Roche CustomBiotech
• Merck KGaA (MilliporeSigma)
• Aldevron (Danaher/Cytiva)
• TriLink BioTechnologies (Maravai)
• Kactus Bio
• Yeasen Biotech
• Takara Bio
• Canvax Biotech
• LGC Biosearch Technologies
• Novoprotein
• Jena Bioscience
• Baseclick GmbH
• Tinzyme
• Promega Corporation
• Kaneka Eurogentec
• BOC Sciences
• Creative Biogene
• HONGENE
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2025-04
Market Dynamics:
Drivers:
The versatility of mRNA platforms has unlocked a broad spectrum of applications, including CRISPR-based gene editing, protein replacement therapies for conditions such as hemophilia and cystic fibrosis, oncology treatments like personalized neoantigen vaccines, and preventive vaccines for infectious diseases such as influenza and COVID-19. Each of these applications relies heavily on GMP-grade IVT enzymes for the synthesis of high-quality RNA drug substances, generating substantial market demand.
As RNA programs transition from preclinical development to commercial-scale production, the need for enzymes optimized for high-yield, continuous IVT processes is rapidly increasing. Key enzymes such as T7 RNA polymerase and capping enzymes are specifically engineered to enable efficient, large-scale transcription at kilogram levels, which crucial for mass vaccine and therapeutic RNA production while also addressing challenges related to cost efficiency and supply chain reliability.
To meet this growing demand, companies are investing in GMP-certified manufacturing facilities, expanding production capabilities, and forming strategic partnerships with CDMOs and biopharma firms to streamline mRNA manufacturing workflows. These initiatives are aimed at ensuring consistent, scalable, and compliant access to GMP-grade IVT enzymes for clinical and commercial applications, thereby reinforcing the critical infrastructure supporting the global therapeutic RNA industry.
Challenges:
GMP-grade enzymes are expensive, which raises the total cost of mRNA manufacturing. This makes it difficult for applications that are sensitive to cost, especially in developing nations or for treatments aimed at rare diseases with limited patient bases. The cost is still an obstacle to wider use, even if developments like KACTUS's MaxPureTM T7 RNA Polymerase are designed to cut costs. The presence of dsRNA complicates regulatory approval and raises purifying costs because it needs to be kept to a minimum to meet safety regulations. Significant R&D expenditure is driven by this technical barrier, which raises production costs and slows market expansion.
Regional Trends:
North America has the largest market share during the forecast period. North America is at the forefront of clinical trials for mRNA-based treatments, such as gene editing (e.g., CRISPR), protein replacement therapies, and cancer immunotherapies. The market is further boosted by these applications, which depend on GMP-grade T7 RNA Polymerase for dependable mRNA synthesis. This collaboration streamlines the production of mRNA therapeutics and strengthens North America's position as a leader in end-to-end mRNA solutions by combining GMP-grade RNA polymerases with sophisticated LNP formulation.
However, Asia-Pacific governments are actively loosening laws to facilitate gene editing and mRNA therapies, creating an atmosphere that is conducive to the use of GMP-grade IVT enzymes. The development of RNA-based medicines in the region has accelerated due to the simplification of clinical trial approval procedures and greater support for biotech innovation in nations like China, Japan, and India.
Asia-Pacific has emerged as a desirable location for the production of GMP-grade IVT enzymes and mRNA therapies due to its cost-effective manufacturing advantages over North America and Europe. Reflecting this trend, Merck, which has a sizable presence in the area, teamed up with Inspirna, Inc. in January 2024 to use GMP-grade IVT enzymes to enhance mRNA-based therapeutics in oncology by utilizing the Asia-Pacific's growing biomanufacturing and research infrastructure.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customization/3248
Recent Developments:
• In April 2024, TriLink BioTechnologies (TriLink), a Maravai LifeSciences company declared its new cGMP mRNA production facility grand opening. Using TriLink's strong mRNA production capabilities, the 32,000-square-foot facility was built especially for mRNA manufacture to assist late-phase drug researchers from Phase 2 to commercialization. As developers swarm to capitalize on the promising technique for an expanding range of applications, the milestone opening is anticipated to contribute to the advancement of mRNA-based therapy. The facility, which is situated in San Diego's Sorrento Valley, has separate Grade C cleanroom suites for the production of mRNA, a capacity increase from 1g to >100g per batch, extensive in-house analytical capabilities, and laboratory space for on-site quality control testing.
• In Mar 2023, Creative Biogene was committed to improving the newest medical technologies, such as vaccines, gene editing, cell treatments, and immunotechnology. In order to support research in the areas of preclinical drug discovery, industrial synthetic application, biomedical development, and fundamental life sciences research, Creative Biogene delivers knowledge to deliver products of the highest quality consistently on time. To further research and project development across multiple domains, Creative Biogene has announced the launch of its GMP-grade mRNA synthesis services.
Global GMP-Grade IVT Enzymes for Therapeutic RNA Market- By Enzyme Type (IVT Workflow)
• RNA Polymerases
o T7 RNA Polymerase (dominant)
o SP6 RNA Polymerase
o T3 RNA Polymerase
• Capping Enzymes
o Vaccinia Capping Enzyme (Cap-0)
o mRNA Cap 2′-O-Methyltransferase (Cap-1)
o Alternative Viral Capping Enzymes (Faustovirus,etc)
• Tailing Enzymes - Poly(A) Polymerase
• Template Generation Enzymes
o Restriction Endonucleases
o High-fidelity DNA Polymerases
• Cleanup / Yield / Protection
o DNase I / dsDNase (RNase-free)
o Inorganic pyrophosphatase
o RNase inhibitor
• Circular RNA Enzymes
o T4 RNA Ligase I/II
o RNase R
Global GMP-Grade IVT Enzymes for Therapeutic RNA Market - By RNA Modality
• mRNA
• saRNA/replicons
• circRNA
• gRNAs/crRNAs (CRISPR)
• Other therapeutic RNAs (lncRNA/antisense where IVT is used)
Global GMP-Grade IVT Enzymes for Therapeutic RNA Market - By Application / Use Stage
• GMP Drug Substance Manufacturing (commercial)
• GMP Clinical Supply & Process Development (Phase I-III)
• Diagnostics/IVD (GMP-qualified enzymes used in regulated kits)
• Translational Research with GMP continuity (pilot/tech-transfer lots)
Global GMP-Grade IVT Enzymes for Therapeutic RNA Market- By End User
• Biopharma/Biotech sponsors (in-house manufacturing)
• CDMOs/CMOs
• Diagnostics companies
• Academic/Government Translational Centers
Global GMP-Grade IVT Enzymes for Therapeutic RNA Market - By Region
North America-
• The US
• Canada
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• Southeast Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Mexico
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of the Middle East and Africa
Read Overview Report- https://www.insightaceanalytic.com/report/gmp-grade-ivt-enzymes-for-therapeutic-rna-market/3248
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 607 400-7072
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP-Grade IVT Enzymes for Therapeutic RNA Market Boosted by Advances in Enzyme Engineering for Improved Yield and RNA Stability here
News-ID: 4253201 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
Automotive Lead Acid Battery Market Strategic Growth Drivers and Outlook 2026 to …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Lead Acid Battery Market Size, Share & Trends Analysis Report By Product (SLI and Micro-Hybrid Batteries), Type (Flooded, Enhanced Flooded, and VRLA), Customer Segment (OEM and Aftermarket), End User (Passenger Car, Light Commercial Vehicles, Heavy Commercial Vehicles, Two-Wheeler, and Three-Wheeler), and Application (Hybrid Vehicles, Electric Vehicles, Light Motor Vehicles, and Heavy Motor Vehicles)- Market…
Automotive Interior Market Investment Opportunities and Forecast 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Interior Market- (By Component Type (Center Stack, Head-up Display, Instrument Cluster, Rear Sear Entertainment, Dome Module, Headliner, Seat, Interior Lighting Door Panel, Center Console, Adhesives & Tapes, Upholstery, Others), By Material (Leather, Fabric, Vinyl, Wood, Glass Fiber Composite, Carbon Fiber Composite, Metal), By Level of Autonomy (Semi-Autonomous, Autonomous, Non-Autonomous),By Electric Vehicle (Battery Electric Vehicle…
Artificial General Intelligence Market Future Landscape and Industry Evolution 2 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Artificial General Intelligence (AGI) Market - (By Type of Offering (Hardware, Software and Service), Type of Technology (Machine Learning, Deep Learning, Natural Language Processing and Robotics), Mode of Deployment (Cloud-based, On Premise and Web-based), Type of AI (Weak AI, Strong AI and Superintelligence), Type of Processing (Image, Text and Voice Processing), Company Size (SMEs and…
Allogenic Cell Therapies Market Revenue Trends and Growth Potential 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Allogenic Cell Therapies Market- by Cell Type(Cardiosphere-Derived Cells (CDCs), Fibroblasts, T-cells, Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs) and Others),Tissue Source(Skin, Blood, PBC, BM and Others), Indication (Acute graft-versus-host disease (GVHD), Chronic Ulcers and Diabetic Foot Ulcers, Osteoarthritis, Crohn's Disease, Cardiovascular Disease, Solid Tumors/Cancers and Others (Alzheimer's Disease, etc.)), Trends, Industry Competition Analysis, Revenue…
More Releases for RNA
CD Formulation Launches Custom Circular RNA Synthesis Service to Accelerate RNA …
CD Formulation introduces a customizable circRNA synthesis service, delivering high-quality, stable circRNAs for therapeutics, vaccines, and gene research, supported by advanced design and QC processes.
CD Formulation, a leading provider of advanced small nucleic acid synthesis [https://www.formulationbio.com/nucleic-acid/custom-small-nucleic-acid-synthesis.html] solutions, is proud to announce the launch of its fully customizable circular RNA (circRNA) synthesis service. This new service addresses the growing need for stable, non-immunogenic RNA molecules for therapeutic development, vaccine research, and…
Self-Amplifying RNA Synthesis Market Gains Traction as Biotech Firms Embrace Sca …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " Self-Amplifying RNA Synthesis Market- (By Product & Service (Products (Enzymes & Reagents, Premade saRNA, Others), Custom Synthesis Services), By Application (Therapeutics Development (Oncology, Infectious Diseases, Others), Biomedical Research), By End-User (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic,…
RNA Extraction and RNA Purification Market: Growth, Trends & Competitive Landsca …
The global RNA Extraction and RNA Purification Market is expected to grow at 6.3% CAGR from 2025 to 2032.
This Market Report is the result of extensive research and analysis conducted by our team of experienced market researchers through -
• 70% efforts of Primary Research
• 15% efforts of Secondary Research
• 15% efforts from the subscription to Paid database providing industry overview, macro and micro economics factors, and financials of private limited…
RNA Targeting Small Molecules Therapeutics Market: Exponential Growth with Risin …
Estimations Predict a CAGR of 29.8% by 2029 in Global RNA Targeting Small Molecules Therapeutics Market Boosted by Precision Medicine, RNA Biomarker Identification and RNA Genetic Manipulation
What Is The Projected Market Size of The Global RNA Targeting Small Molecules Therapeutics Market And Its Growth Rate?
• The market will grow from $6.1 billion in 2024 to $7.87 billion in 2025 at a compound annual growth rate (CAGR) of 28.9%.
• Expected exponential…
Global DNARNA Extraction Kit Market by Type (Cell-free DNA (cfDNA), Sequence-spe …
"DNARNA Extraction Kit Market" is segmented by Company, Region (country), By Type, Application, stakeholders and other participants. This report provides an analysis of revenue and forecast across Type and Application segments for 2023-2032.
The market for DNARNA Extraction Kits has been thoroughly researched via primary and secondary sources to produce this research study. Along with a competitive analysis of the market, segmented by application, type, and geographical trends, it offers a…
Cancer RNA Expression Market to Reap Excessive Revenues by 2028(By sequencing te …
Worldwide cancer is one of the leading cause of death and effective way of treating it still looks unaccomplished in most parts of the world. The factors which influence the successful treatment of cancer are different depending on the stage of diagnosis, treatment availability and availability of trained healthcare professionals coupled with high economic burden of the disease. The gene expression of cancerous cells varies by cancer type and may…