Press release
Biliary Tract Cancer Pipeline Drugs Insights Report 2025: Emerging Therapies and Innovation Trends by DelveInsight
DelveInsight's, "Biliary Tract Cancers Pipeline Insights 2025" report provides comprehensive insights about 80+ companies and 80+ pipeline drugs in Biliary Tract Cancers pipeline landscape. It covers the Biliary Tract Cancer pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Biliary Tract Cancer therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Stay ahead with the latest insights! Download DelveInsight's comprehensive Biliary Tract Cancer Pipeline Report to explore emerging therapies, key companies, and future treatment landscapes @ Biliary Tract Cancer Pipeline Outlook Report [https://www.delveinsight.com/sample-request/biliary-tract-cancers-btcs-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Biliary Tract Cancer Pipeline Report
* On 23 October 2025, AstraZeneca conducted a study is to evaluate the safety, tolerability, PK, immunogenicity, pharmacodynamics, and preliminary efficacy of AZD4360 in adult participants with locally advanced or metastatic solid tumours selected for expression of CLDN18.2.
* On 22 October 2025, Jazz Pharmaceuticals announced a study is to evaluate the efficacy and safety of Zanidatamab plus CisGem (Cisplatin and Gemcitabine) with or without the addition of a programmed death protein 1/ligand-1 (PD-1/L1) inhibitor (physician's choice of either Durvalumab or Pembrolizumab, where approved under local regulations) as first line of treatment for participants with human epidermal growth factor receptor 2 (HER2)-positive biliary tract cancer.
* On 20 October 2025, Seagen initiated a clinical trial studies how well tucatinib works for solid tumors that make either more HER2 or a different type of HER2 than usual (HER2 alterations) The solid tumors studied in this trial have either spread to other parts of the body (metastatic) or cannot be removed completely with surgery (unresectable).
* DelveInsight's Biliary Tract Cancer pipeline report depicts a robust space with 80+ active players working to develop 80+ pipeline therapies for Biliary Tract Cancer treatment.
* The leading Biliary Tract Cancer Companies such as Yantai Rongchang Pharmaceutical, RemeGen, EMD Serono, SMT Bio Co., Ltd., CSPC ZhongQi Pharmaceutical Technology Co., Ltd., InnoPharmax Inc., Lee's Pharmaceutical Limited, Hoffmann-La Roche, Shanghai Miracogen Inc., Zymeworks Inc., Bristol-Myers Squibb, Eli Lilly and Company, Ipsen, Threshold Pharmaceuticals, Leap Therapeutics, Hutchison Medipharma Limited, Array BioPharma, Redx Pharma Plc, Compass Therapeutics, Lee's Pharmaceutical Limited and others.
* Promising Biliary Tract Cancer Therapies such as PEMAZYRE (pemigatinib), ROZLYTREK (entrectinib), VITRAKVI (larotrectinib), TIBSOVO (ivosidenib), LYTGOBI (futibatinib), KEYTRUDA (pembrolizumab), IMFINZI (durvalumab), TAFINLAR (dabrafenib) + MEKINIST (trametinib), and others.
Discover how the Biliary Tract Cancer Treatment paradigm is evolving. Access DelveInsight's in-depth Biliary Tract Cancer Pipeline Analysis for a closer look at promising breakthroughs @ Biliary Tract Cancer Clinical Trials and Studies [https://www.delveinsight.com/sample-request/biliary-tract-cancers-btcs-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Biliary Tract Cancer Overview
Biliary tract cancer is a cancer that forms in the cells of the bile ducts, gallbladder or ampulla of Vater. Cancer of the bile ducts is called cholangiocarcinoma and is classified depending on which part of the bile duct the cancer develops into intrahepatic (affects bile ducts within the liver), hilar (occurs at the junction of the left and right hepatic ducts) and extrahepatic (affects the common bile duct outside the liver) sharing different genetic, risk factors and clinical presentation.
Biliary tract cancer: Emerging Drugs Profile
* MRG 002: Miracogen
MRG 002, is an antibody-drug conjugate (ADC) composed of a humanized immunoglobulin G1 (IgG1) monoclonal antibody directed against the tumor-associated antigen (TAA) human epidermal growth factor receptor 2 conjugated to the microtubule-disrupting cytotoxic agent monomethyl auristatin E (MMAE), with potential antineoplastic activity. An open-label, single-arm, multi-center, phase II Clinical Study of MRG002 are conducted by Miracogen for the treatment of patients with HER2-positive unresectable, locally advanced or metastatic biliary tract cancer. MRG002 is administrated by an IV infusion of 2.6 mg/kg on Day 1 of every 3 weeks (21-day cycle).
* Disitamab vedotin: Yantai Rongchang Pharmaceutical
Disitamab Vedotin is an antibody-drug conjugate with a drug structure consisting of three parts Anti-human epidermal growth factor receptor 2 extracellular domain (HER2 ECD) antibody; Linker (MC-Val-Cit-PAB, Linker); and Cytotoxic Monomethyl Auristatin E (Monomethyl Auristatin E, MMAE). This product is white to light yellow loose body, after reconstitution, it is colorless to light yellow clear liquid.
* Envafolimab: Alphamab Oncology
Envafolimab is a PD-L1 single-domain antibody Fc fusion protein independently developed by Alphamab. Based on the unique design, Envafolimab has advantages in safety, convenience and compliance, and can be used for patients who are not suitable for intravenous infusion with a lower medical cost. On March 30, 2020, Alphamab, 3D Medicines and Simcere reached a three-way strategic collaboration. Alphamab, as the original research party, is responsible for production and quality, 3D Medicines is responsible for global clinical development in the field of oncology, registration and commercialization abroad, and Simcere is responsible for the exclusive commercial promotion of the product in mainland China. At present, Envafolimab is being evaluated in clinical trials for multiple cancer indications in China, the United States and Japan, and the research for multiple indications have entered the registration/clinical Phase III. Envafolimab has been awarded orphan drug designation (ODD) by FDA in the United States for the treatment of advanced biliary tract cancer.
* DKN-01: Leap Therapeutics
DKN-01 is a humanized monoclonal antibody that binds to and blocks the activity of the Dickkopf-1 (DKK1) protein. DKK1 modulates the Wnt/Beta-catenin and PI3kinase/AKT signaling pathways and has an important role in promoting tumor proliferation, metastasis, angiogenesis, and in mediating an immune suppressive tumor microenvironment through enhancing the activity of myeloid-derived suppressor cells and downregulating NK cell ligands on tumor cells. The U.S. Food and Drug Administration has granted DKN-01 Orphan Drug Designation for the treatment of gastric and gastroesophageal junction cancer and Fast Track Designation in combination with tislelizumab for the treatment of patients with gastric and gastroesophageal junction adenocarcinoma whose tumors express high DKK1 protein, following disease progression on or after prior fluoropyrimidine- and platinum- containing chemotherapy and if appropriate, human epidermal receptor growth factor (HER2)/neu-targeted therapy. Currently the drug is in Phase II of development.
Get a detailed analysis of the latest innovations in the Biliary Tract Cancer pipeline. Explore DelveInsight's expert-driven report today! @ Biliary Tract Cancer Unmet Needs [https://www.delveinsight.com/sample-request/biliary-tract-cancers-btcs-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Biliary Tract Cancer Companies
Yantai Rongchang Pharmaceutical, RemeGen, EMD Serono, SMT Bio Co., Ltd., CSPC ZhongQi Pharmaceutical Technology Co., Ltd., InnoPharmax Inc., Lee's Pharmaceutical Limited, Hoffmann-La Roche, Shanghai Miracogen Inc., Zymeworks Inc., Bristol-Myers Squibb, Eli Lilly and Company, Ipsen, Threshold Pharmaceuticals, Leap Therapeutics, Hutchison Medipharma Limited, Array BioPharma, Redx Pharma Plc, Compass Therapeutics, Lee's Pharmaceutical Limited and others.
Biliary Tract Cancer pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
* Intravenous
* Subcutaneous
* Oral
* Intramuscular
Biliary Tract Cancer Products have been categorized under various Molecule types such as
* Monoclonal antibody
* Small molecule
* Peptide
Download DelveInsight's latest report to gain strategic insights into upcoming Biliary Tract Cancer Therapies and key Biliary Tract Cancer Developments @ Biliary Tract Cancer Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/biliary-tract-cancers-btcs-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Biliary Tract Cancer Pipeline Report
* Coverage- Global
* Biliary Tract Cancer Companies- Yantai Rongchang Pharmaceutical, RemeGen, EMD Serono, SMT Bio Co., Ltd., CSPC ZhongQi Pharmaceutical Technology Co., Ltd., InnoPharmax Inc., Lee's Pharmaceutical Limited, Hoffmann-La Roche, Shanghai Miracogen Inc., Zymeworks Inc., Bristol-Myers Squibb, Eli Lilly and Company, Ipsen, Threshold Pharmaceuticals, Leap Therapeutics, Hutchison Medipharma Limited, Array BioPharma, Redx Pharma Plc, Compass Therapeutics, Lee's Pharmaceutical Limited and others.
* Biliary Tract Cancer Therapies- PEMAZYRE (pemigatinib), ROZLYTREK (entrectinib), VITRAKVI (larotrectinib), TIBSOVO (ivosidenib), LYTGOBI (futibatinib), KEYTRUDA (pembrolizumab), IMFINZI (durvalumab), TAFINLAR (dabrafenib) + MEKINIST (trametinib) , and others.
* Biliary Tract Cancer Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Biliary Tract Cancer Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Which companies are leading the race in Biliary Tract Cancer drug development? Find out in DelveInsight's exclusive Biliary Tract Cancer Pipeline Report-access it now! @ Biliary Tract Cancer Emerging Drugs and Major Companies [https://www.delveinsight.com/sample-request/biliary-tract-cancers-btcs-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Contents
* Introduction
* Executive Summary
* Biliary Tract Cancer: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Biliary Tract Cancer- DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* Envafolimab: Alphamab Oncology
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II/III)
* SMT-NK: SMT bio Co., Ltd.
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* HA121-28: CSPC ZhongQi Pharmaceutical Technology
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I)
* ZKAB001: Lee's Pharmaceutical Limited
* Drug profiles in the detailed report.....
* Preclinical and Discovery Stage Products
* Drug name: Company name
* Drug profiles in the detailed report.....
* Inactive Products
* Biliary Tract Cancer Key Companies
* Biliary Tract Cancer Key Products
* Biliary Tract Cancer- Unmet Needs
* Biliary Tract Cancer- Market Drivers and Barriers
* Biliary Tract Cancer- Future Perspectives and Conclusion
* Biliary Tract Cancer Analyst Views
* Biliary Tract Cancer Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=biliary-tract-cancer-pipeline-drugs-insights-report-2025-emerging-therapies-and-innovation-trends-by-delveinsight]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/report-store/biliary-tract-cancers-btcs-pipeline-insight
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biliary Tract Cancer Pipeline Drugs Insights Report 2025: Emerging Therapies and Innovation Trends by DelveInsight here
News-ID: 4241422 • Views: …
More Releases from ABNewswire

Windy City Chimney Heroes Announces Expanded Service Areas Across the Greater Ch …
Windy City Chimney Heroes, a long-standing chimney company established in 1997, has expanded its service areas across the Greater Chicago region. The company now serves Chicago, Evanston, Oak Park, Naperville, Schaumburg, Cicero, Skokie, Berwyn, and Arlington Heights. Owner Wesley Cook says the expansion will help meet rising demand for reliable chimney repair and inspection services.
Windy City Chimney Heroes, a trusted chimney repair and maintenance provider founded in 1997, has announced…

Injury 2 Wellness Centers Promotes Wellness Care Through Ongoing Chiropractic Su …
As the year draws to a close and many individuals begin setting health goals for the new year, Injury 2 Wellness Centers is encouraging Georgia residents to prioritize wellness care through consistent chiropractic treatment. Far beyond addressing back or neck pain, chiropractic care can serve as a foundation for long-term physical health, improved function, and overall quality of life.
Decatur, GA - December 10, 2025 - As the year draws to…

Avalon Tree Services Reminds Atlanta Homeowners to Prioritize Winter Tree Care f …
As temperatures drop and trees enter their dormant season, Avalon Tree Services is reminding homeowners across Greater Atlanta that winter is one of the most important times to invest in proper tree care. Cold weather, ice accumulation, and seasonal storms can all take a toll on tree health and stability-making preventative maintenance essential for safety and long-term growth.
Atlanta, GA - December 10, 2025 - As temperatures drop and trees enter…
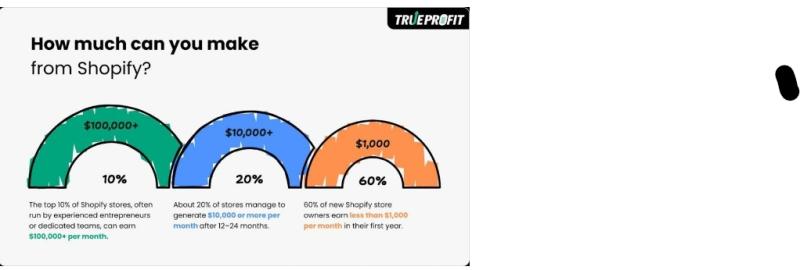
TrueProfit Shares 2026 Guidance for Shopify Startups to Focus on Profit First No …
As Shopify heads into 2026 with millions of active stores worldwide and continued growth in global ecommerce, TrueProfit - a Net Profit Analytics platform for Shopify merchants - is urging new founders to treat profitability as a starting requirement, not an afterthought.
With a low barrier to entry and a mature app ecosystem, Shopify remains one of the easiest ways to launch an online business. But TrueProfit's latest briefing on early-stage…
More Releases for Biliary
Global Metal Pancreatic Biliary Stent Market Size by Application, Type, and Geog …
According to Market Research Intellect, the global Metal Pancreatic Biliary Stent market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The metal pancreatic biliary stent market is witnessing consistent growth due to the rising prevalence of…
Major Market Share Shift in Biliary Catheters Industry: Advancements In Biliary …
What Is the Forecasted Market Size and Growth Rate for the Biliary Catheters Market?
The market size for biliary catheters has experienced robust growth in recent years. There is an expected growth from $3.42 billion in 2024 to $3.75 billion in 2025, corresponding to a compound annual growth rate (CAGR) of 9.7%. Factors like increased occurrences of biliary tract ailments, the popularity of minimally invasive procedures, a growing aging population, more…
Primary Biliary Cholangitis Treatment Market
Primary Biliary Cholangitis Treatment Market
Global Primary Biliary Cholangitis Treatment Market Expected to Reach US$ YY Million by 2030, Growing at a CAGR of YY%: Market Insights and Dynamics
The Global Primary Biliary Cholangitis Treatment Market, which reached US$ YY billion in 2022, is anticipated to achieve US$ YY million by 2030, exhibiting a robust CAGR of YY% during the forecast period from 2024 to 2031.
Primary Biliary Cholangitis (PBC), formerly known as…
Biliary Stents Market - Unleashing Flow: Empowering Biliary Health with Innovati …
Newark, New Castle, USA - new report, titled Biliary Stents Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Biliary Stents market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Biliary Stents market. The report offers an overview of the market, which…
High Prevalence of Biliary Diseases Across the Globe is Driving the Biliary Sten …
Stent is referred as plastic or metal tube inserted in human body into lumen or duct to keep the passage open. Various types of stents such as expandable stents and simple plastic stents are used for various purposes. Different types of stents such as coronary, vascular, and biliary stents are employed for various purposes. ‘Biliary’ is referred to the bile duct, which is a long tube-like structure carrying bile fluid.…
Rise in Surgical Procedures of Biliary Diseases is Expected to Boost the Biliary …
Stent is referred as plastic or metal tube inserted in human body into lumen or duct to keep the passage open. Various types of stents such as expandable stents and simple plastic stents are used for various purposes. Different types of stents such as coronary, vascular, and biliary stents are employed for various purposes. ‘Biliary’ is referred to the bile duct, which is a long tube-like structure carrying bile fluid.…
