Press release
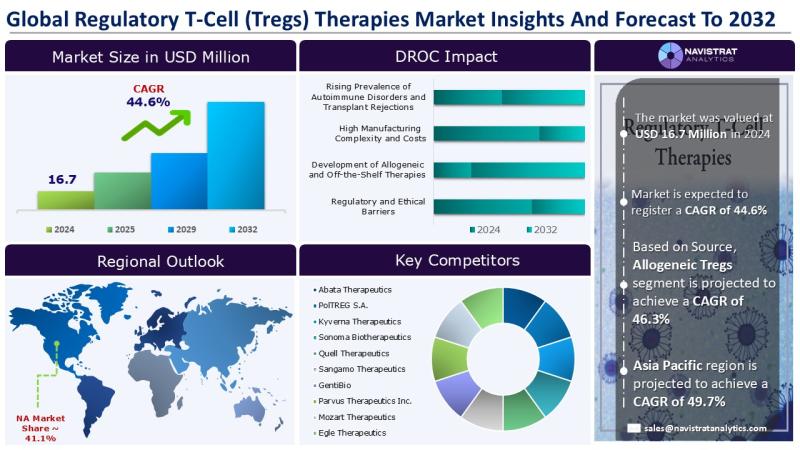
Regulatory T-Cell (Tregs) Therapies Market Size to Reach USD 320.4 million in 2032
Regulatory T Cells, Treg Therapies, Immunotherapy, Market Trends, Market Forecast, Company Profile, Competitive Landscape
Request free copy of this report: https://navistratanalytics.com/request-free-sample/1547
October 09, 2025- The growing incidence of autoimmune disorders and transplant rejections is a major factor driving revenue growth in the Regulatory T-Cell (Treg) therapies market. Data from the Lupus Foundation of America indicates that lupus affects about 1.5 million people in the U.S. and at least five million worldwide, with 90% of cases occurring in women. Most patients are diagnosed between ages 15 and 44, and systemic lupus represents more than 70% of all cases.
In March 2023, Sonoma Biotherapeutics, Inc., and Regeneron Pharmaceuticals, Inc. announced a collaboration to leverage their combined scientific expertise and technology platforms to develop novel Treg therapies for autoimmune diseases. This partnership integrates Regeneron's VelociSuite technologies for discovering and characterizing fully human antibodies and T cell receptors (TCRs) with Sonoma's capabilities in Treg development.
Treg therapies hold significant promise for treating autoimmune diseases, preventing transplant rejection, and managing inflammatory conditions by harnessing the body's natural immune regulators. However, they present greater challenges than other adoptive cell therapies like CAR-T. The limited availability of Tregs and the critical need for highly pure cell populations make their development and manufacturing particularly complex.
Want to Know What's Fueling the Regulatory T-Cell (Tregs) Therapies Market Growth? Get Exclusive Report Insights Here: https://navistratanalytics.com/report_store/regulatory-t-cell-tregs-therapies-market/
Segments market overview and growth Insights
Based on source, the Regulatory T-Cell (Tregs) therapies market is segmented into Autologous Tregs and Allogeneic Tregs. The Autologous Tregs segment accounted for the largest market share in 2024. In September 2024, PolTREG S.A., a clinical-stage biotechnology company focused on cellular therapies for autoimmune diseases, announced the launch of a study evaluating CAR-Treg cells in a humanized mouse model of neuroinflammatory disease. These CAR-Treg cells are derived from PolTREG's PTG-007 Treg cells, an autologous polyclonal candidate showing promise for the treatment of type 1 diabetes.
Regional market overview and growth insights
North America held the largest market share in the Regulatory T-Cell (Tregs) Therapies market in 2024. The market is being driven by the growing prevalence of autoimmune disorders and transplant rejections, along with advancements in cell therapy and immunomodulation. Data from the Autoimmune Association indicate that autoimmune diseases impact over 50 million Americans, representing 8% of the U.S. population, and prevalence continues to rise. Epidemiological studies further reveal that the global incidence of autoimmune diseases has been increasing by 19.1% annually, with rheumatological conditions such as Sjögren's syndrome and lupus rising by 7.1% each year.
Competitive Landscape and Key Competitors
The Regulatory T-Cell (Tregs) Therapies market is characterized by a fragmented structure, with many competitors holding a significant share of the market. list of major players included in the Regulatory T-Cell (Tregs) Therapies market report are:
o Abata Therapeutics
o PolTREG S.A.
o Kyverna Therapeutics
o Sonoma Biotherapeutics
o Quell Therapeutics
o Sangamo Therapeutics
o GentiBio
o Parvus Therapeutics Inc.
o Mozart Therapeutics
o Egle Therapeutics
o Nektar Therapeutics
o Coya Therapeutics, Inc
o TR1X, Inc.
o Egle Therapeutics
o Cugene, Inc.
Buy Your Exclusive Copy Now: https://navistratanalytics.com/purchase-report/1547
Major strategic developments by leading competitors
Abata Therapeutics: In August 2024, Abata Therapeutics, a company focused on advancing Treg therapies for severe autoimmune and inflammatory diseases, announced an equity investment from Bristol Myers Squibb to support the development of its Treg cell therapy programs. The funding will enable Abata to advance its pipeline, with clinical trials of ABA-101 in progressive multiple sclerosis expected to start soon.
Kyverna Therapeutics: In August 2023, Kyverna Therapeutics, a clinical-stage cell therapy company focused on developing a new generation of treatments for autoimmune diseases, announced the successful completion of an oversubscribed USD 60 million Series B extension, raising the total Series B funding to USD 145 million. The proceeds will support Kyverna's clinical trials in the U.S. and Europe, accelerating progress toward delivering transformative and life-saving therapies to patients.
Unlock the Key to Transforming Your Business Strategy with Our Regulatory T-Cell (Tregs) Therapies Market Insights -
• Download the report summary: https://navistratanalytics.com/request-free-sample/1547
• Request customization: https://navistratanalytics.com/request-customization/1547
Navistrat Analytics has segmented the Regulatory T-Cell (Tregs) therapies market based on source, therapeutic type, indication, and end-use, and region:
• Source Outlook (Revenue, USD Million; 2022-2032)
o Autologous Tregs
o Allogeneic Tregs
• Therapeutic Type Outlook (Revenue, USD Million; 2022-2032)
o Polyclonal Tregs
o Antigen-Specific Tregs
o CAR-Tregs (Chimeric Antigen Receptor Tregs)
o Engineered Tregs
• Indication Outlook (Revenue, USD Million; 2022-2032)
o Autoimmune Diseases
o Transplantation
o Graft-Versus-Host Disease (GvHD)
o Inflammatory and Allergic Disorders
o Oncology
o Others
• End-Use Outlook (Revenue, USD Million; 2022-2032)
o Hospitals and Clinics
o Pharmaceutical and Biotechnology Companies
o Research Institutes and Academic Centers
o Contract Research Organizations (CROs)
• Regional Outlook (Revenue, USD Million; 2022-2032)
o North America
a. U.S.
b. Canada
c. Mexico
o Europe
a. Germany
b. France
c. U.K.
d. Italy
e. Spain
f. Benelux
g. Nordic Countries
h. Rest of Europe
o Asia Pacific
a. China
b. India
c. Japan
d. South Korea
e. Oceania
f. ASEAN Countries
g. Rest of APAC
o Latin America
a. Brazil
b. Rest of LATAM
o Middle East & Africa
a. GCC Countries
b. South Africa
c. Israel
d. Turkey
e. Rest of MEA
Get a preview of the complete research study: https://navistratanalytics.com/report_store/regulatory-t-cell-tregs-therapies-market/
Navistrat Analytics
Visit Us: www.navistratanalytics.com
Email Us: Sales@navistratanalytics.com
Asia-Pacific: +91-9073010653
Follow Us LinkedIn: https://www.linkedin.com/company/navistrat-analytics/
Address - Salt Lake, West Bengal, India, 700091
At Navistrat Analytics, we provide high-quality, comprehensive syndicated and customized market research reports that deliver actionable insights and empower businesses through data-driven strategies. Choose Navistrat Analytics as your strategic growth partner for reliable market intelligence, and let us help you navigate the complexities of the market with clarity, precision, and confidence.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory T-Cell (Tregs) Therapies Market Size to Reach USD 320.4 million in 2032 here
News-ID: 4216399 • Views: …
More Releases from Navistrat Analytics
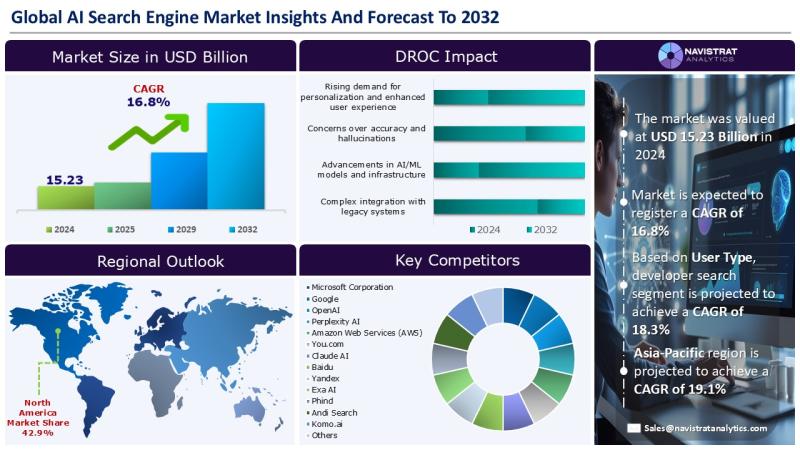
AI Search Engine Market Size to Reach USD 51.48 Billion in 2032
The AI Search Engine market was valued at USD 15.23 Billion in 2024 and is expected to register a revenue CAGR of 16.8%.
January 20, 2026 - Exponential growth of digital content is a major driver of revenue growth in the AI search engine market. The rapid increase in online articles, videos, images, social media content, and enterprise data makes it difficult for traditional search methods to deliver accurate and…
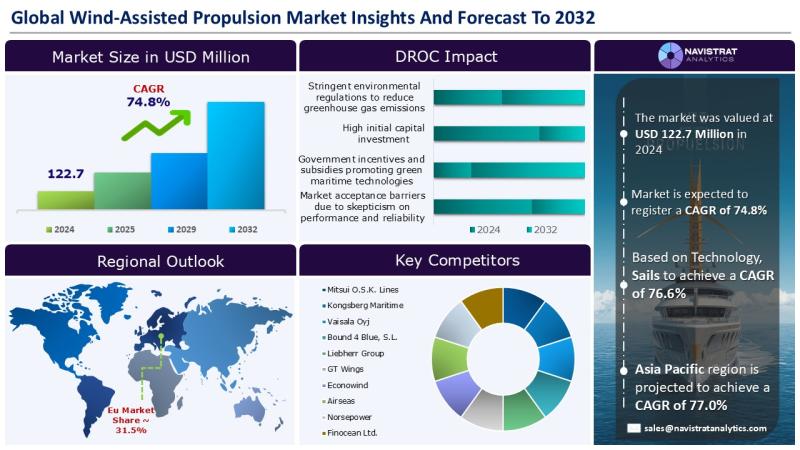
Wind-Assisted Propulsion Market Size to Reach USD 10,727.4 million in 2032
The Wind-Assisted Propulsion market, valued at USD 122.7 million in 2024, is expected to register revenue CAGR of 74.8%.
Request free copy of this report: https://navistratanalytics.com/request-free-sample/1747
December 20, 2025- Stringent environmental regulations aimed at cutting greenhouse gas (GHG) emissions are a major factor driving revenue growth in the wind-assisted propulsion (WAP) market. The maritime sector is undergoing a transformative shift toward sustainability as it seeks to reduce its carbon footprint. According…
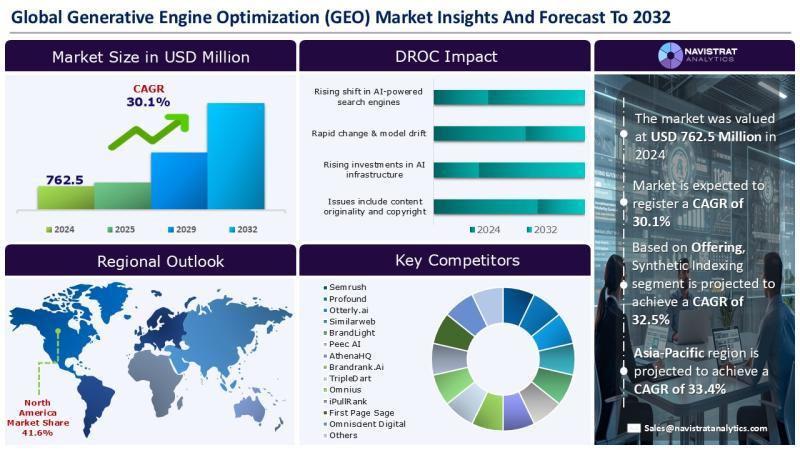
Generative Engine Optimization (GEO) Market Size to Reach USD 6,067.7 Million in …
Generative Engine Optimization (GEO) Market Size to Reach USD 6,067.7 Million in 2032
November 19, 2025 - Growing demand for zero-click or low-click experiences among users is a major driver for the revenue growth of Generative Engine Optimization (GEO) market. AI search engines and generative AI platforms increasingly deliver summarized, conversational responses, compelling businesses to optimize their content for visibility within these answer-rich interfaces rather than traditional search listings. This shift…
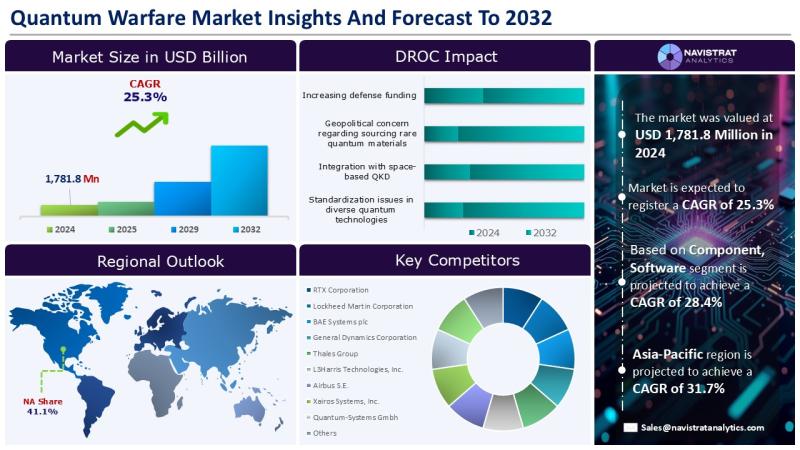
Quantum warfare market is USD 1.78 billion in 2024 and is projected to register …
8th September 2025 - Quantum technologies, which include cyber security, quantum sensing, and secure communications, are becoming increasingly important for future military and economic dominance. According to the World Economic Forum (WEF), 17 nations have already invested in national quantum, whereas over 150 countries lack a quantum strategy. Major economies throughout the world are investing extensively in diverse R&D initiatives to improve their technical dominance in important areas.
Defense departments…
More Releases for Treg
Treg Cell-Based Therapies Clinical Trial Landscape Expands With 55+ Emerging The …
The "Treg Cell-Based Therapies Pipeline Insight" report delivers a detailed assessment of therapeutic programs spanning the entire development continuum, from early discovery and preclinical research to advanced clinical-stage candidates. It features comprehensive drug profiles highlighting mechanisms of action, clinical trial progress, regulatory milestones, and partnerships, funding activities, and enabling technology platforms influencing product development.
DelveInsight's most recent evaluation underscores the accelerated global momentum in the Treg Cell-Based Therapies domain, with over…
Treg Therapy Market is Set to Experience a Revolutionary Growth
Regulatory T cells (Tregs) are a specialized subset of T cells that play a crucial role in maintaining immune homeostasis by suppressing excessive immune responses and preventing autoimmune diseases. Treg therapy, which involves the modulation or expansion of Tregs to restore immune balance, is emerging as a promising treatment for various autoimmune diseases, transplant rejection, and inflammatory disorders. Given their ability to control immune responses, Treg-based therapies hold great potential…
Tr1 Treg Treatment to Be Assessed in a Crohn's Disease Trial
The global Treg Therapy Market is rapidly gaining momentum as regulatory T-cell (Treg) therapies emerge as a transformative approach in immunology. Tregs are a specialized subset of T-cells that maintain immune tolerance and homeostasis, preventing the body from attacking its own tissues. Harnessing these cells offers a promising strategy to treat autoimmune diseases, organ transplant rejection, and chronic inflammatory conditions.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/72661
With advancements…
Treg Cell-Based Therapies Pipeline 2025: Latest FDA Approvals, Clinical Trials, …
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Treg Cell-Based Therapies pipeline constitutes key 51+ companies continuously working towards developing 55+ Treg Cell-Based Therapies treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
The Treg Cell-Based Therapies Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The…
Treg Cell-Based Therapies Pipeline 2025: FDA Updates, Therapy Innovations, and C …
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Treg Cell-Based Therapies pipeline constitutes key 51+ companies continuously working towards developing 55+ Treg Cell-Based Therapies treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
The Treg Cell-Based Therapies Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also…
Treg Cell-Based Therapies Clinical Trials Analysis 2025: EMA, PDMA, FDA Approval …
(Albany, USA) DelveInsight's "Treg Cell-Based Therapies Pipeline Insight, 2025" comprehensively analyzes the current clinical landscape and growth prospects in the Treg cell-based therapies market. The report covers disease insights, treatment guidelines, and a detailed pipeline assessment from preclinical to marketed stages. It includes drug mechanisms, clinical studies, regulatory progress, and key developments such as collaborations, mergers, funding, and designations.
For emerging Treg cell-based therapies drugs, the Treg cell-based therapies pipeline analysis…