Press release
Global Rare Disease Clinical Trials Market Growth Accelerates: Strategic Forecast Predicts $19.44 Billion by 2029
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.What Will the Rare Disease Clinical Trials Industry Market Size Be by 2025?
In recent years, there has been a notable increase in the size of the rare disease clinical trials market. It is projected to rise from $12.06 billion in 2024 to $13.30 billion in 2025, indicating a compound annual growth rate (CAGR) of 10.3%. Factors contributing to the growth during the historic period include heightened awareness of genetic disorders among healthcare providers, elevated demand for detailed patient supervision during trials, advancements in developing personalized medicine methods, an inclination towards patient-centric trial methodologies, and the escalating necessity for worldwide, multi-center clinical studies.
What's the Long-Term Growth Forecast for the Rare Disease Clinical Trials Market Size Through 2029?
Anticipated to experience hasty growth in the forthcoming years, the market size of rare disease clinical trials is set to surge to $19.45 billion by 2029, achieving a compound annual growth rate (CAGR) of 10.0%. The expected growth within this forecast period is linked to several factors such as the inclusion of more rare disease treatments in clinical trials, a surge in regulatory aid for approving orphan drugs, a boost in disease-specific patient registries and databases, an escalation in international clinical trials and multi-center investigations, and a rise in funding initiatives directed towards infrastructure for rare disease research. The forecast period will also see major trends such as the enhanced use of decentralized trial models, progressive approaches towards patient recruitment, integrating real-world evidence into trial plans, progression towards precision medicine strategies, and a growing embrace of digital health technologies.
View the full report here:
https://www.thebusinessresearchcompany.com/report/rare-disease-clinical-trials-global-market-report
What Are the Key Growth Drivers Fueling the Rare Disease Clinical Trials Market Expansion?
The advancement of personalized medicine is anticipated to fuel the growth of the rare disease clinical trials market in the future. Personalized medicine is a concept that refers to customizing medical treatment to fit the unique attributes of each patient, including genetic makeup, lifestyle, and environmental factors. This expansion in personalized medicine is attributed to the enhanced outcomes of treatments, since it customizes therapies to match each patient's individual profile, thereby enhancing efficacy and safety. Rare disease clinical trials contribute to the growth of personalized medicine by producing patient-specific insights that steer the creation of targeted treatments designed for each person's distinct genetic and molecular profile. For example, the Food and Drug Administration (FDA) reported in February 2024, according to the Personalized Medicine Coalition (PMC), a nonprofit organization based in the US, that personalized medicines accounted for 34% of approvals in 2022, which rose to 38% in 2023. As a result, the advancement of personalized medicine is accelerating the growth of the rare disease clinical trials market.
Get your free sample here:
https://www.thebusinessresearchcompany.com/sample.aspx?id=28311&type=smp
What Are the Key Trends Driving Rare Disease Clinical Trials Market Growth?
Leading businesses in the rare disease clinical trials market are dedicated to driving innovation, such as utilizing a customer-oriented strategy to deliver bespoke trial designs, increase patient participation, and speed up targeted treatments for unusual ailments. A customer-oriented strategy implies meeting the requirements, aspirations, and preferences of biotech and pharmaceutical customers by delivering customized and effective clinical trial solutions. For instance, Evestia Clinical Limited, an American pharmaceutical research firm, was unveiled as the new brand identity for EMAS Pharma in March 2025. This rebranding signifies a strategic effort to expedite the company's international growth. The company is staunch in its mission to hasten the development of treatments for rare diseases by offering expert-led, customized clinical trial solutions tailored to these diseases' unique trials. This focus forms a strong part of the company's brand and services.
How Is the Rare Disease Clinical Trials Market Segmented?
The rare disease clinical trials market covered in this report is segmented as
1) By Phase: Phase I, Phase II, Phase III, Phase IV
2) By Therapeutic Area: Oncology, Cardiovascular Disorders, Neurological Disorders, Infectious Disease, Genetic Disorders, Autoimmune And Inflammation, Hematologic Disorders, Musculoskeletal Disorders, Other Therapeutic Areas
3) By Study Design: Interventional, Observational, Expanded Access
4) By End-User: Pharmaceutical Companies, Biotechnology Companies, Research Institutes, Other End-Users
Subsegments:
1) By Phase I: First In Human Trials, Dose Escalation Studies, Safety And Tolerability Studies
2) By Phase II: Proof Of Concept Studies, Dose Response Studies, Efficacy And Safety Studies
3) By Phase III: Randomized Controlled Trials, Comparative Effectiveness Studies, Large Scale Multicenter Trials
4) By Phase IV: Post Marketing Surveillance, Long Term Safety Studies, Real World Evidence Studies
Tailor your insights and customize the full report here:
https://www.thebusinessresearchcompany.com/customise?id=28311&type=smp
Which Companies Are Leading the Charge in Rare Disease Clinical Trials Market Innovation?
Major companies operating in the rare disease clinical trials market are F. Hoffmann-La Roche AG, Pfizer Inc., Novartis AG, AstraZeneca plc, Takeda Pharmaceutical Company Limited, IQVIA Holdings Inc., Laboratory Corporation of America Holdings, ICON plc, Moderna Inc., Charles River Laboratories International Inc., Parexel International Corporation, Revvity Inc., Sarepta Therapeutics Inc., TFS HealthScience, Inventiva S.A., Tonix Pharmaceuticals Holding Corp., SpringWorks Therapeutics Inc., OrphAI Therapeutics Inc., BBCR Consulting LLC, and Credevo Inc.
Which Regions Are Leading the Global Rare Disease Clinical Trials Market in Revenue?
North America was the largest region in the rare disease clinical trials market in 2024. The regions covered in rare disease clinical trials report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
Purchase the full report today:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=28311
This Report Supports:
1.Business Leaders & Investors - To identify growth opportunities, assess risks, and guide strategic decisions.
2.Manufacturers & Suppliers - To understand market trends, customer demand, and competitive positioning.
3.Policy Makers & Regulators - To track industry developments and align regulatory frameworks.
4.Consultants & Analysts - To support market entry, expansion strategies, and client advisory work.
Connect with us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company,
Twitter: https://twitter.com/tbrc_info,
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ.
Contact Us
Saumya Sahey
Europe: +44 7882 955267,
Asia: +44 7882 955267 & +91 8897263534,
Americas: +1 310-496-7795
Email: saumyas@tbrc.info
Learn More About The Business Research Company
With over 15,000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Our flagship product, the Global Market Model delivers comprehensive and updated forecasts to support informed decision-making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Rare Disease Clinical Trials Market Growth Accelerates: Strategic Forecast Predicts $19.44 Billion by 2029 here
News-ID: 4215347 • Views: …
More Releases from The Business Research Company
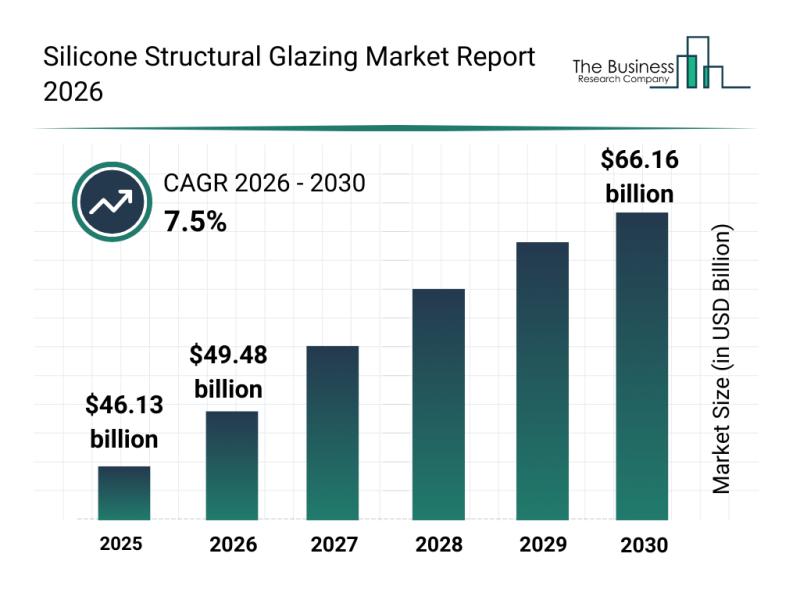
Leading Companies Solidify Their Presence in the Silicone Structural Glazing Mar …
The silicone structural glazing market is positioned for significant expansion in the coming years, driven by advances in building technology and increased environmental awareness. This sector is evolving rapidly as demand grows for more energy-efficient and aesthetically appealing architectural solutions. Let's explore the market's current size, key players, emerging trends, and the main segments that are shaping its future.
Silicone Structural Glazing Market Value Forecast Through 2030
The market for silicone…
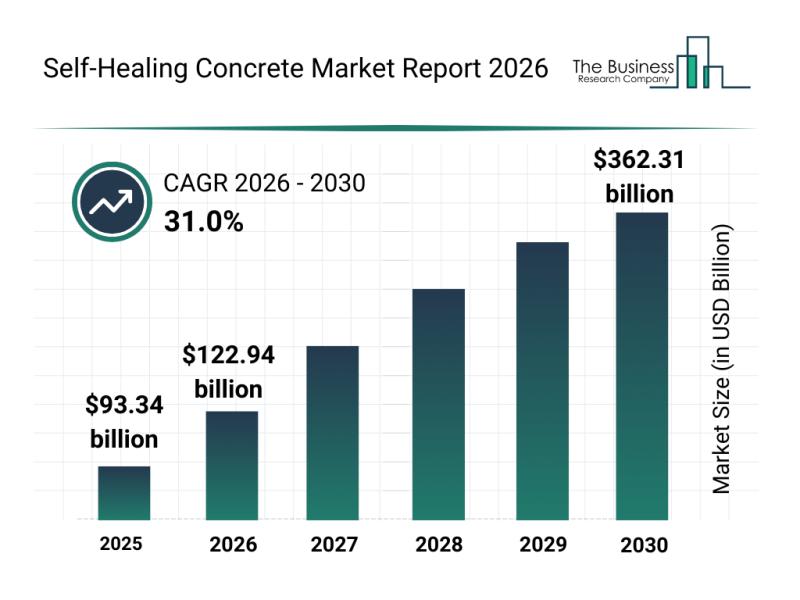
Future Prospects: Key Trends Shaping the Self-Healing Concrete Market up to 2030
The self-healing concrete market is capturing significant attention as innovations and sustainability demands rise in construction. This sector is set to experience remarkable growth due to advancements in materials and technology, shaping the future of durable and intelligent infrastructure solutions. Let's explore the market's size, key players, emerging trends, and segment outlook to understand its trajectory.
Projected Market Size and Growth Prospects for the Self-Healing Concrete Market
The self-healing concrete market…
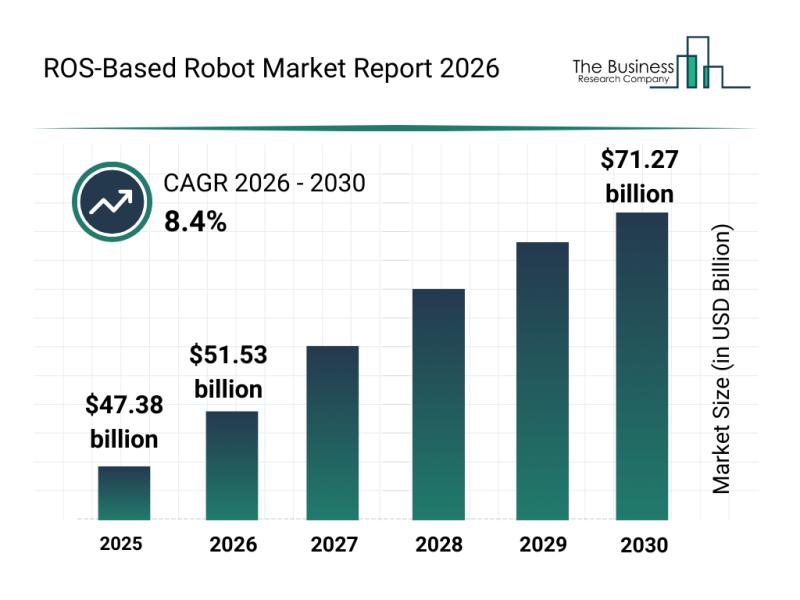
Analysis of Key Market Segments Driving the ROS-Based Robot Industry
The ROS-based robot market is positioned for substantial growth as robotics technology continues to advance rapidly. With increasing innovation in software, hardware, and AI integration, this sector is set to transform multiple industries by 2030. Below, we explore the market's future size, leading companies, key trends, and segmentation details to understand its evolving landscape.
Projected Market Size and Expansion of the ROS-Based Robot Market
The ROS-based robot market is anticipated to…
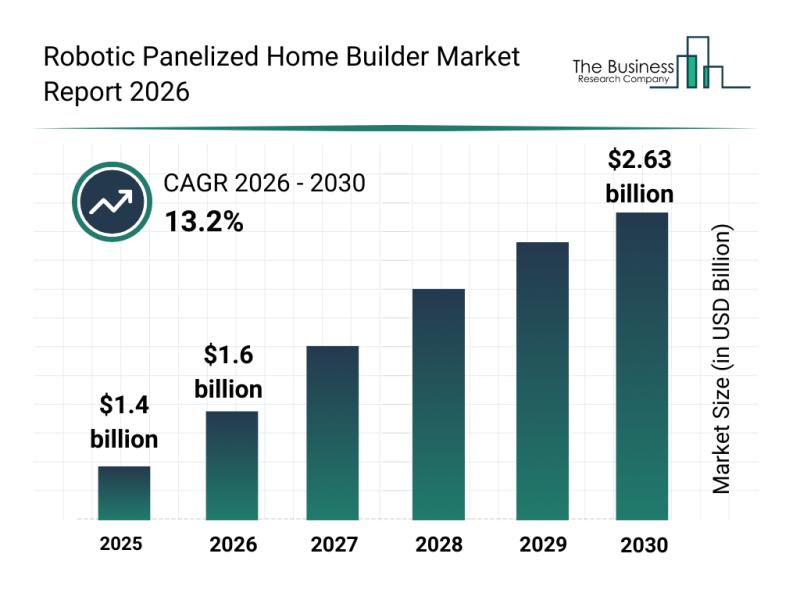
Global Trends Overview: The Rapid Evolution of the Robotic Panelized Home Builde …
The robotic panelized home builder market is positioned for impressive growth in the coming years as automation and robotics increasingly transform construction processes. Driven by technological advancements and expanding prefab housing projects, this market is set to reshape how homes are built with greater speed and efficiency. Let's explore the market's size, leading companies, emerging trends, and key segments that are shaping its future.
Strong Growth Forecast for the Robotic Panelized…
More Releases for Trials
Clinical Trials Management System Market Trends: How Decentralized Clinical Tria …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical Device Firms, CROs)- Market Outlook And Industry Analysis 2031"
The global Clinical Trials Management System market is estimated to reach…
Brazil Clinical Trials Market ANVISA Brazil Guidelines Brazil Clinical Trials Re …
Brazil Cancer Drugs Clinical Trials Insight 2024 Report Offering:
• Brazil Clinical Trials Market Opportunity 2024 and 2030 (In US$ Billion)
• Clinical Trials Regulatory Framework In Brazil
• Total Number of Cancer Drugs In Clinical Trials In Brazil
• Total Number Of Cancer Drugs Approved In Brazil
• 400 Pages Clinical Trials Insight On All Cancer Drugs In Clinical Trials By Company, Indication and Phase
• 80 Pages Clinical Insight On All Cancer Drugs Approved in Market By Company and Indication
• Insight…
Clinical Trials Management System Market Clinical Trials Management System Marke …
InsightAce Analytic announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical Device Firms, CROs)- Market Outlook And Industry Analysis 2031.
The global Clinical Trials Management System market is estimated to reach over USD…
Revolutionizing Clinical Trials: The Evolution of eCOA, eSource, and the Clinica …
The global eCOA, eSource & clinical trials market is valued at US$ 48 billion in 2023 and is expected to reach a market size of US$ 104 billion by the end of 2033, expanding rapidly at a CAGR of 8% over the next ten years. Worldwide demand for eCOA (electronic clinical outcome assessment) solutions is predicted to rise at a CAGR of 8.2% over the forecast period.
In the dynamic landscape…
Virtual Clinical Trials Market - Bridging the Gap: Virtual Clinical Trials Revol …
Newark, New Castle, USA - The latest report from Growth Plus Reports analyzes the production, potential applications, demand, major manufacturers, and SWOT analysis of the global Virtual Clinical Trials Market.
Virtual Clinical Trials Market: https://www.growthplusreports.com/report/virtual-clinical-trials-market/9106
The Virtual Clinical Trials Market Report assists in determining the optimum distribution methods for certain products as well as possible markets for future product launches. The report also analyses the purchase and supply trends that influence the…
Clinical Trials Market: Coronavirus Pandemic Pushes Sponsors, Patients to Adopt …
Clinical trials Market: Introduction
According to the report, the global clinical trials market was valued over US$ 46.7 Bn in 2019 and is projected to expand at a CAGR of ~5% from 2020 to 2030. High prevalence and increase in incidence rate of chronic diseases, and rise in R&D activities in biotechnology & pharmaceuticals industries are anticipated to drive the global clinical trials market from 2020 to 2030. North America is projected to…
