Press release
Artificial Intelligence Clinical Trial Protocol Feasibility Tool Market Poised to Hit $2.17 Billion by 2029 with Accelerating Growth Trends
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.Artificial Intelligence Clinical Trial Protocol Feasibility Tool Market Size Growth Forecast: What to Expect by 2025?
The market for tools facilitating the feasibility of clinical trial protocols through artificial intelligence has seen an unparalleled surge of late. It's projected to bolster from $0.65 billion in 2024 to $0.83 billion in 2025, indicating a compound annual growth rate (CAGR) of 27.5%. The ascent witnessed in this historical phase draws its roots from the escalating intricacies of clinical trial directives, an increased mass of clinical trial data, the soaring expenses of conventional clinical trials, heightened utilization of digital health files, and an amplified emphasis on trials revolving around patient welfare.
How Will the Artificial Intelligence Clinical Trial Protocol Feasibility Tool Market Size Evolve and Grow by 2029?
In the upcoming years, the market size of the artificial intelligence clinical trial protocol feasibility tool is predicted to experience massive growth. It is forecasted to rise to a value of $2.17 billion in 2029 with a compound annual growth rate (CAGR) of 27.1%. The surge anticipated in the forecast period can be ascribed to the burgeoning demand for personalized medicine trials, the escalating adoption of decentralized clinical trials, a rise in the utilization of natural language processing, expanding focus on patient diversity in trials, and growing significance of real-time feasibility monitoring. Fundamental trends during the forecast period comprise the development of adaptive algorithms for optimizing site selection, incorporating genomic data to facilitate precision trial protocols, innovating automated trial cost and timeline estimation, creating tools to boost patient diversity in clinical trials, and integrating multi-source real-world evidence for accurate feasibility.
View the full report here:
https://www.thebusinessresearchcompany.com/report/artificial-intelligence-clinical-trial-protocol-feasibility-tool-global-market-report
What Drivers Are Propelling the Growth of Artificial Intelligence Clinical Trial Protocol Feasibility Tool Market Forward?
As the focus on precision medicine gains traction, it's anticipated to fuel the expansion of the market for artificial intelligence clinical trial protocol feasibility tools. Precision medicine is a healthcare strategy tailored to the patient's genetic makeup, environment, and lifestyle for more targeted and efficacious treatments. The growing interest in this field is attributed to advancements in genomic sequencing and biomarker identification, which allow for accurate detection and tailored treatments. AI clinical trial protocol feasibility tools enhance precision medicine, assisting in data-driven trial designs that are optimal for specific patient groups. These tools minimize trial inefficiencies by providing predictions for patient recruitment, timeframes, and risks, thereby enhancing the precision and results of clinical research. For example, Novotech, an Australian biotechnology firm, confirmed in March 2024 that in 2023, 43% of the 217 FDA-approved oncology treatments were precision oncology treatments, with 78 featuring DNA or NGS-detectable biomarkers. Therefore, the burgeoning focus on precision medicine is a significant driver for the growth of the AI clinical trial protocol feasibility tool market.
Get your free sample here:
https://www.thebusinessresearchcompany.com/sample.aspx?id=28142&type=smp
Which Emerging Trends Are Transforming the Artificial Intelligence Clinical Trial Protocol Feasibility Tool Market in 2025?
Significant players in the market of artificial intelligence clinical trial protocol feasibility tools are concentrating on the creation of innovative solutions such as software for planning trials based on predictive analytics. This helps to improve the precision of protocol design and reduce delays in trials. This software solution utilises both real-time and historical clinical trial data to predict timelines, pinpoint potential risks, and refine study designs to make trials more cost-effective and efficient. For example, in June 2024, Lokavant Inc., an American clinical intelligence company, introduced Spectrum. This was the first tool for assessing the feasibility of clinical trials using AI, with the specific aim of optimising trials from the strategic planning stage to the mid-study re-forecasting stage. The platform houses software for planning trials based on predictive analytics, allowing for ongoing, real-time feasibility studies, along with the prediction of costs and timelines, and financial forecasting without the requirement for manual input. Spectrum also offers dynamic, real-time (re)forecasting by merging live trial data, allowing study groups to check progress, examine different potential scenarios, and improve enrolment results. Furthermore, this tool offers highly accurate estimations for costs, timelines, and operational risks, facilitating quicker, data-based decision making for the entire length of the clinical trial lifecycle.
What Are the Key Segments in the Artificial Intelligence Clinical Trial Protocol Feasibility Tool Market?
The artificial intelligence clinical trial protocol feasibility tool market covered in this report is segmented as
1) By Component: Software, Services
2) By Deployment Mode: Cloud-Based, On-Premises
3) By Application: Protocol Design, Site Selection, Patient Recruitment, Risk Assessment, Other Applications
4) By End-User: Pharmaceutical And Biotechnology Companies, Contract Research Organizations, Academic And Research Institutes, Other End-Users
Subsegments:
1) By Software: Predictive Analytics, Machine Learning, Natural Language Processing, Data Integration
2) By Services: Consulting, Implementation, Training And Support, Managed Services
Tailor your insights and customize the full report here:
https://www.thebusinessresearchcompany.com/customise?id=28142&type=smp
Who Are the Key Players Shaping the Artificial Intelligence Clinical Trial Protocol Feasibility Tool Market's Competitive Landscape?
Major companies operating in the artificial intelligence clinical trial protocol feasibility tool market are IQVIA Holdings Inc., SAS Institute Inc., Veeva Systems Inc., Tempus AI Inc., Cytel Inc., ArisGlobal LLC., Saama Technologies Inc., Norstella, ConcertAI, Komodo Health Inc., H1 Inc., TriNetX Inc., ObjectiveHealth LLC, Lokavant Inc., Inato Inc., Clinerion Ltd., Faro Health Inc., Lantern Pharma Inc., Ryght AI, and BEKHealth Corporation.
What Geographic Markets Are Powering Growth in the Artificial Intelligence Clinical Trial Protocol Feasibility Tool Market?
North America was the largest region in the artificial intelligence clinical trial protocol feasibility tool market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in artificial intelligence clinical trial protocol feasibility tool report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
Purchase the full report today:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=28142
This Report Supports:
1.Business Leaders & Investors - To identify growth opportunities, assess risks, and guide strategic decisions.
2.Manufacturers & Suppliers - To understand market trends, customer demand, and competitive positioning.
3.Policy Makers & Regulators - To track industry developments and align regulatory frameworks.
4.Consultants & Analysts - To support market entry, expansion strategies, and client advisory work.
Connect with us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company,
Twitter: https://twitter.com/tbrc_info,
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ.
Contact Us
Saumya Sahey
Europe: +44 7882 955267,
Asia: +44 7882 955267 & +91 8897263534,
Americas: +1 310-496-7795
Email: saumyas@tbrc.info
Learn More About The Business Research Company
With over 15,000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Our flagship product, the Global Market Model delivers comprehensive and updated forecasts to support informed decision-making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Artificial Intelligence Clinical Trial Protocol Feasibility Tool Market Poised to Hit $2.17 Billion by 2029 with Accelerating Growth Trends here
News-ID: 4214334 • Views: …
More Releases from The Business Research Company
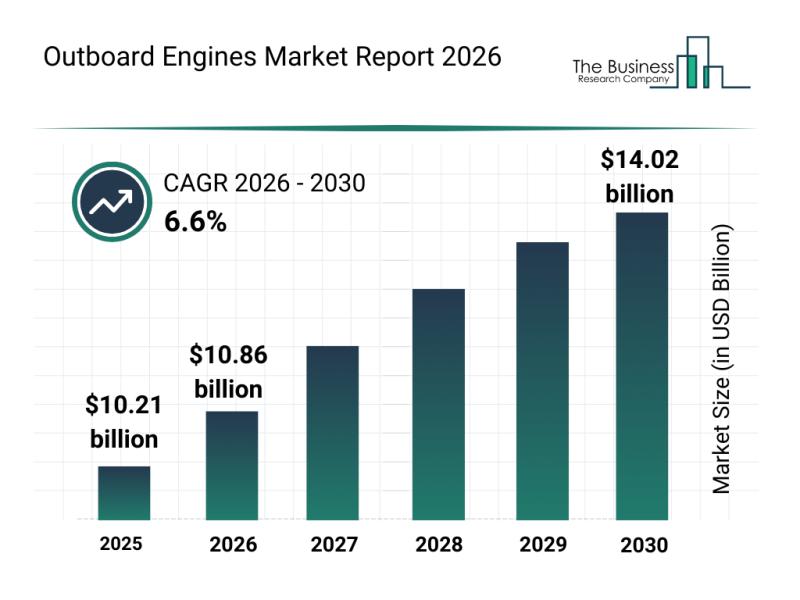
Leading Companies Advancing Innovation and Growth in the Outboard Engines Market
The outboard engines market is positioned for significant growth in the coming years, driven by advancements in technology and changing consumer preferences. The evolving landscape highlights key innovations and strategic moves by industry leaders to capitalize on emerging opportunities. Let's explore the market size projections, leading companies, future trends, and segmentation details shaping this industry.
Expected Expansion of the Outboard Engines Market by 2030
The outboard engines market is projected…
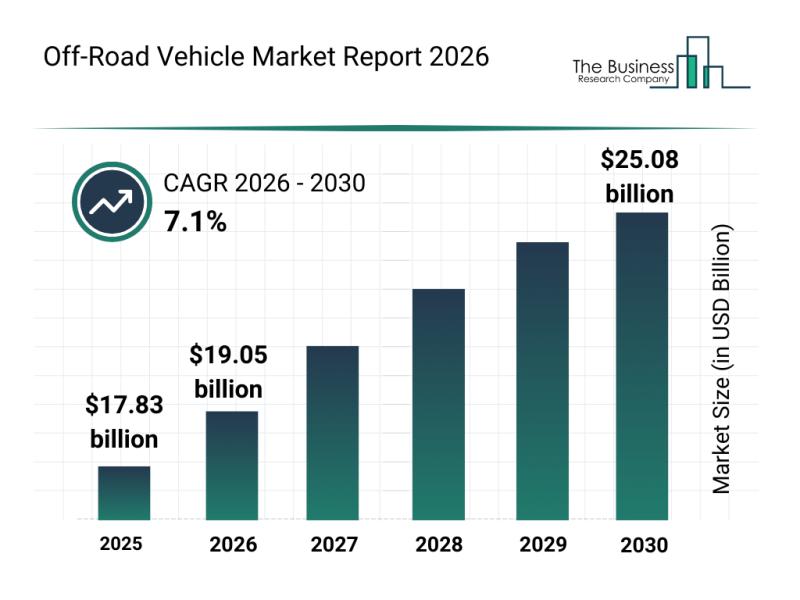
Growth Patterns, Market Segmentation, and Competitive Approaches Influencing the …
The off-road vehicle market is steadily gaining traction as demand rises across multiple sectors. Innovations in technology, growing interest in outdoor adventure, and strategic defense initiatives are all contributing to a promising outlook for this dynamic industry. Let's explore the market's size, key players, emerging trends, and segmentation to better understand what lies ahead.
Projected Expansion and Market Size of the Off-Road Vehicle Market
The off-road vehicle industry is set…
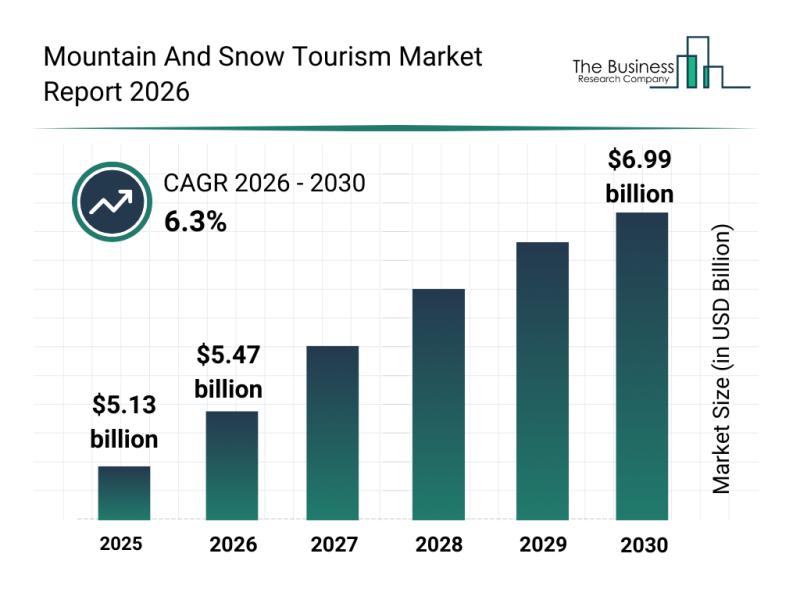
Leading Companies Enhancing Their Presence in the Mountain and Snow Tourism Mark …
The mountain and snow tourism sector is gaining considerable momentum as travelers seek unique and immersive outdoor experiences. With evolving preferences and technological advancements, this industry is set to grow significantly over the coming years. Let's explore the current market size, leading players, emerging trends, and key segments shaping this exciting travel niche.
Projected Market Size Growth in Mountain and Snow Tourism
The mountain and snow tourism market is poised…
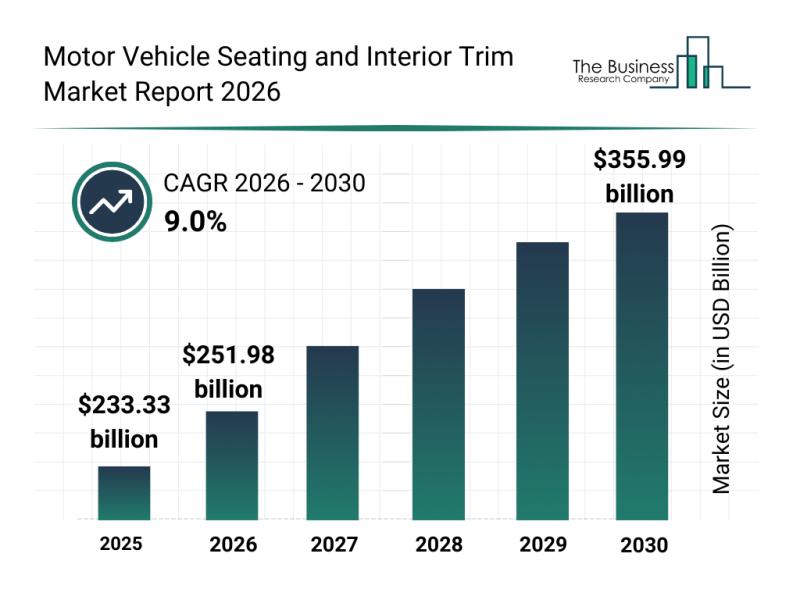
Future Perspectives: Key Trends Shaping the Motor Vehicle Seating and Interior T …
The motor vehicle seating and interior trim sector is poised for impressive growth over the next several years. As automotive design evolves and consumer preferences shift, this market is expected to expand substantially, driven by several notable trends and innovations. Below, we explore the market's size, major players, key growth drivers, and segmentation details to provide a comprehensive view of what lies ahead.
Projected Expansion of the Motor Vehicle Seating and…
More Releases for Protocol
Effective Scientific Writing - The Paper Protocol
Stefan Lang, medical writer and trainer for scientific writing, published his new guidebook for scientists 'The Paper Protocol - Systematic Instructions for Writing a Biomedical Research Paper', which is now available in online bookstores.
"Scientists are trained to conduct their research efficiently with the help of experimental and clinical protocols. How helpful would it be to have a systematic writing protocol that helps them afterwards to put their research results on…
DeSpace Protocol Investor Announcement
DeSpace Protocol has partnered with a new team of strategic investors
LONDON, September 7, 2021 – Today we’re extremely proud to introduce some of the strategic investors that are backing DeSpace. We’re honored to have Lead Wallet ( https://www.leadwallet.io/ ), OMNI ( https://omni.ai/ ), Almora Capital ( https://www.almora.capital/ ), Criterion ( https://criterionvc.com/ ), Dach Capital ( https://dach.capital/ ) , and BTA Ventures ( https://bta.ventures/ ) partnering with DeSpace to help…
Routing Protocol and MPLS Project
Metaswitch Project Details
Product Brief
The product is Virtualized Network Appliance that supports multiple service functions like IP Routing, IPSEC VPN, Deep Packet Inspection, VoIP Gateway across multiple nodes in a cluster. It used a customized forwarding-plane over Intel-DPDK and Metaswitch Routing Stack. Each node could have both Data-plane and Control-plane functionality.
• DC-OSPF, DC-BGP, DC-RTM Integration with Distributed Interface Manager
Interfaces were owned by Intel-DPDK and a proprietary Distributed Interface Manager managed them. I3…
Website and HTTP/2 protocol?
Performance is key
Need for speed, we all want everything faster & better. The same applies to websites and users expectations. They don't want to wait for the web page to load, they want their user experience (UX) to be smooth, web content to be served without delay. Latency is killing your conversions!
Amazon found every 100ms of latency could cost them 1% in sales. For Google delay of 0.5s when displaying…
Multi Protocol Labeled Switching (MPLS) Internet Protocol (IP) Virtual Private N …
Multi protocol labeled switching (MPLS) is the mechanism used in high performance telecommunication networks and is considered to be highly scalable data transferring method from one network node to the other. It belongs to the packet switched networks family where the data is in the form of packets that are to be transferred. Each data packet is assigned with a particular label which alone influences the packet routing or forwarding…
CANopen Protocol stacks for Fujitsu MB9BF506n
Merseburg, 04/25/2013 – emtas GmbH located in the heart of Germany has extended its Master and Slave CANopen stacks to support the Fujitsu Semiconductor MB9BF506n microcontroller from the FM3 family. This efficient controller is based on the ARM Cortex-M3 with a clock rate up to 80MHz using 2 integrated CAN-controller of the widely used C_CAN from Bosch. This combination of devices is an ideal base for CANopen applications using…
