Press release
Graft versus Host Disease Pipeline Drugs Report 2025: Promising Research Updates, Evolving Therapeutics, and Future Healthcare Strategies
DelveInsight's, "Graft versus host disease Pipeline Insight 2025" report provides comprehensive insights about 45+ Graft Versus Host Disease Companies and 50+ pipeline drugs in Graft versus host disease pipeline landscape. It covers the Graft Versus Host Disease pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Graft versus Host Disease Pipeline Therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Curious about the latest updates in the Graft versus Host Disease Pipeline? Click here to explore the therapies and trials making headlines @ Graft versus Host Disease Pipeline Outlook Report [https://www.delveinsight.com/sample-request/graft-versus-host-disease-gvhd-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Graft versus Host Disease Pipeline Report
* On 16 September 2025, Novartis Pharmaceuticals conducted a study is to assess the efficacy and safety of ruxolitinib therapy in Chinese adults and adolescents ( greater than or equal to 12 years old) with Grade II-IV steroid-refractory acute graft versus host disease (SR-aGvHD).
* On 15 September 2025, Deciphera Pharmaceuticals LLC announced a study is to determine if vimseltinib is safe, tolerable and works effectively to treat adults with active moderate to severe cGVHD. Participants will be treated with vimseltinib in 28-day treatment cycles for approximately 2 years.
* On 15 September 2025, Syndax Pharmaceuticals organized a phase 1/2, Open-Label, Dose Escalation and Dose Expansion Study to Evaluate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamic Activity, and Efficacy of SNDX- 6352 in Subjects With Active Chronic Graft Versus Host Disease Who Have Received at Least 2 Lines of Prior Therapy.
* DelveInsight's Graft Versus Host Disease pipeline report depicts a robust space with 45+ active players working to develop 50+ pipeline therapies for Graft Versus Host Disease treatment.
* The leading Graft Versus Host Disease Companies such as Abbisko Therapeutics, Equillium, Theriva Biologics, Seres Therapeutics, CytoMed Therapeutics, Beijing Tide Pharmaceutical Co., Ltd, CTI BioPharma, ViGenCell Inc., Lipella Pharmaceuticals, Cellestia Biotech, Jiangsu HengRui Medicine Therapeutics, Genentech, AltruBio, Orca Bio, GSK, Amgen and others.
* Promising Graft Versus Host Disease Therapies such as Ibrutinib, Prednisone, ruxolitinib, Axatilimab, GDC-8264, Ruxolitinib, Defibrotide , and others.
Want to know which companies are leading innovation in Graft versus Host Disease? Dive into the full pipeline insights @ Graft versus Host Disease Clinical Trials Assessment [https://www.delveinsight.com/sample-request/graft-versus-host-disease-gvhd-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
The Graft versus Host Disease Pipeline Report provides disease overview, pipeline scenario and therapeutic assessment of the key pipeline therapies in this domain. The Graft versus Host Disease Pipeline Report also highlights the unmet needs with respect to the Graft versus Host Disease.
Graft versus Host Disease Overview
Graft versus host disease (GVHD) is a serious complication that occurs when donor immune cells attack the recipient's body following an allogeneic stem cell or bone marrow transplant. This condition arises because the donor's immune cells, recognizing the recipient's cells as foreign, initiate an immune response against them. GVHD primarily involves T-cells from the donor graft and can be classified into two main types: acute and chronic. Acute GVHD typically develops within the first 100 days post-transplant, manifesting through symptoms like skin rashes, jaundice, liver enzyme abnormalities, and diarrhea.
Graft versus host disease Emerging Drugs
* Itolizumab: Equillium
Itolizumab is a clinical-stage, first-in-class monoclonal antibody that selectively targets the CD6-ALCAM pathway. This pathway plays a central role in modulating the activity and trafficking of T cells that drive a number of immuno-inflammatory diseases. Itolizumab was launched in India in 2013 under the brand name ALZUMAB. Itolizumab received emergency use approval in India to treat cytokine release syndrome in COVID-19 patients with moderate to severe acute respiratory distress syndrome. The drug is currently in Phase III clinical development for the treatment of acute graft-versus-host disease (aGVHD).
* ABSK021: Abbisko Therapeutics
Pimicotinib (ABSK021), which was independently developed by Abbisko Therapeutics, is a novel, orally administered, highly selective and potent small-molecule inhibitor of CSF-1R. Abbisko is actively exploring the potential of pimicotinib in treating other indications including many types of solid tumors in clinic, and has obtained approval from NMPA to conduct a Phase II clinical study in chronic graft-versus-host disease. Currently, the drug is in the Phase II stage of its development for the treatment of Chronic Graft Versus Host Disease.
* SYN-004: Theriva Biologics
SYN-004 (ribaxamase) is an oral prophylactic therapy designed to degrade certain IV beta-lactam antibiotics within the GI tract and maintain the natural balance of the gut microbiome for acute graft-versus-host-disease (aGVHD) in allogeneic hematopoietic cell transplant (HCT) recipients. Allogeneic HCT recipients routinely receive long courses of IV beta-lactam antibiotics to treat infection. Antibiotic-mediated damage of the gut microbiome in allogeneic HCT recipients has been strongly associated with adverse outcomes including CDI, vancomycin-resistant enterococci (VRE) colonization and potentially fatal bacteremia and aGVHD. Currently, the drug is in the Phase I/II stage of its development for the treatment of Graft versus host disease.
* SER-155: Seres Therapeutics
SER-155 is an oral, investigational therapeutic comprising a fermented consortium of commensal bacteria, specifically designed to support immunocompromised patients. Its primary mechanism of action involves augmenting crucial microbiome functions that contribute to improved survival and reduced risks of infections and graft versus host disease (GvHD) in patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT). By leveraging insights from human clinical data, SER-155 aims to fortify the gut microbiome, enhance immune function, and protect against gastrointestinal infections and bacteremia. This multifaceted approach may offer a promising solution for patients facing the challenges of stem cell transplantation. Currently, the drug is in Phase I stage of its clinical trial for the treatment of GvHD.
If you're tracking ongoing Graft versus Host Disease Clinical trials, this press release is a must-read. Tap to see the breakthroughs @ Graft versus Host Disease Treatment Drugs [https://www.delveinsight.com/sample-request/graft-versus-host-disease-gvhd-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Graft versus Host Disease Companies
Abbisko Therapeutics, Equillium, Theriva Biologics, Seres Therapeutics, CytoMed Therapeutics, Beijing Tide Pharmaceutical Co., Ltd, CTI BioPharma, ViGenCell Inc., Lipella Pharmaceuticals, Cellestia Biotech, Jiangsu HengRui Medicine Therapeutics, Genentech, AltruBio, Orca Bio, GSK, Amgen and others.
The Graft versus Host Disease Pipeline report provides insights into
* The report provides detailed insights about companies that are developing therapies for the treatment of Graft versus Host Disease with aggregate therapies developed by each company for the same.
* It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Graft versus Host Disease Treatment.
* Graft versus Host Disease Companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
* Graft versus Host Disease Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
* Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Graft versus Host Disease market.
GVHD Pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Graft versus host disease products have been categorized under various ROAs such as
* Intravenous
* Subcutaneous
* Oral
* Intramuscular
Graft versus host disease Products have been categorized under various Molecule types such as
* Monoclonal antibody
* Small molecule
* Peptide
From emerging drug candidates to competitive intelligence, the Graft versus Host Disease Pipeline Report covers it all - check it out now @ Graft versus Host Disease Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/graft-versus-host-disease-gvhd-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Graft versus Host Disease Pipeline Report
* Coverage- Global
* Graft versus Host Disease Companies- Abbisko Therapeutics, Equillium, Theriva Biologics, Seres Therapeutics, CytoMed Therapeutics, Beijing Tide Pharmaceutical Co., Ltd, CTI BioPharma, ViGenCell Inc., Lipella Pharmaceuticals, Cellestia Biotech, Jiangsu HengRui Medicine Therapeutics, Genentech, AltruBio, Orca Bio, GSK, Amgen and others.
* Graft Versus Host Disease Therapies- Ibrutinib, Prednisone, ruxolitinib, Axatilimab, GDC-8264, Ruxolitinib, Defibrotide , and others.
* Graft versus Host Disease Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Graft versus Host Disease Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Stay ahead in Healthcare Research - discover what's next for the Graft versus Host Disease treatment landscape in this detailed analysis @ Graft versus Host Disease Emerging Drugs and Major Players [https://www.delveinsight.com/sample-request/graft-versus-host-disease-gvhd-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Contents
* Introduction
* Executive Summary
* Graft versus host disease: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Graft versus host disease- DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* Itolizumab: Equillium
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* ABSK021: Abbisko Therapeutics
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I)
* SER-155: Seres Therapeutics
* Drug profiles in the detailed report.....
* Preclinical and Discovery Stage Products
* Drug name: Company name
* Drug profiles in the detailed report.....
* Inactive Products
* Graft versus host disease Key Companies
* Graft versus host disease Key Products
* Graft versus host disease- Unmet Needs
* Graft versus host disease- Market Drivers and Barriers
* Graft versus host disease- Future Perspectives and Conclusion
* Graft versus host disease Analyst Views
* Graft versus host disease Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=graft-versus-host-disease-pipeline-drugs-report-2025-promising-research-updates-evolving-therapeutics-and-future-healthcare-strategies]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/report-store/graft-versus-host-disease-gvhd-pipeline-insight
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Graft versus Host Disease Pipeline Drugs Report 2025: Promising Research Updates, Evolving Therapeutics, and Future Healthcare Strategies here
News-ID: 4187175 • Views: …
More Releases from ABNewswire

Windy City Chimney Heroes Announces Expanded Service Areas Across the Greater Ch …
Windy City Chimney Heroes, a long-standing chimney company established in 1997, has expanded its service areas across the Greater Chicago region. The company now serves Chicago, Evanston, Oak Park, Naperville, Schaumburg, Cicero, Skokie, Berwyn, and Arlington Heights. Owner Wesley Cook says the expansion will help meet rising demand for reliable chimney repair and inspection services.
Windy City Chimney Heroes, a trusted chimney repair and maintenance provider founded in 1997, has announced…

Injury 2 Wellness Centers Promotes Wellness Care Through Ongoing Chiropractic Su …
As the year draws to a close and many individuals begin setting health goals for the new year, Injury 2 Wellness Centers is encouraging Georgia residents to prioritize wellness care through consistent chiropractic treatment. Far beyond addressing back or neck pain, chiropractic care can serve as a foundation for long-term physical health, improved function, and overall quality of life.
Decatur, GA - December 10, 2025 - As the year draws to…

Avalon Tree Services Reminds Atlanta Homeowners to Prioritize Winter Tree Care f …
As temperatures drop and trees enter their dormant season, Avalon Tree Services is reminding homeowners across Greater Atlanta that winter is one of the most important times to invest in proper tree care. Cold weather, ice accumulation, and seasonal storms can all take a toll on tree health and stability-making preventative maintenance essential for safety and long-term growth.
Atlanta, GA - December 10, 2025 - As temperatures drop and trees enter…
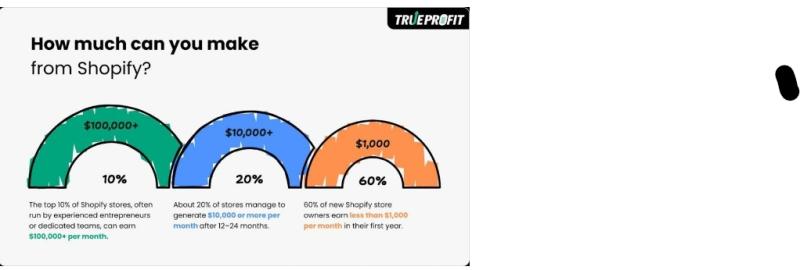
TrueProfit Shares 2026 Guidance for Shopify Startups to Focus on Profit First No …
As Shopify heads into 2026 with millions of active stores worldwide and continued growth in global ecommerce, TrueProfit - a Net Profit Analytics platform for Shopify merchants - is urging new founders to treat profitability as a starting requirement, not an afterthought.
With a low barrier to entry and a mature app ecosystem, Shopify remains one of the easiest ways to launch an online business. But TrueProfit's latest briefing on early-stage…
More Releases for Graft
Bone Graft and Graft Substitute Market Latest Research, Top Impacting Factors, G …
The "Bone Graft and Graft Substitute Market Analysis to 2028" is a specialized and in-depth study of the healthcare industry with a special focus on the global market trend analysis. A bone graft is a surgical treatment used to repair problems with bones or joints. Bone grafting, or transplanting of bone tissue, is useful in fixing bones that are impaired from trauma or problem joints. Bone growth around an embedded…
Synthetic Bioactive Bone Graft Substitutes Market Growing need for bone graft su …
Global Synthetic Bioactive Bone Graft Substitutes Market Overview:
The Synthetic Bioactive Bone Graft Substitutes market is a broad category that includes a wide range of products and services related to various industries. This market comprises companies that operate in areas such as consumer goods, technology, healthcare, and finance, among others.
In recent years, the Synthetic Bioactive Bone Graft Substitutes market has experienced significant growth, driven by factors such as increasing consumer demand,…
Bone Graft Substitutes Market Size to Hit $3.8 Billion by 2028 | Bone Graft Subs …
Market Overview:
According to our experience research team, Bone Graft Substitutes Market was valued at USD 2.7 Billion in 2021, and the global Bone Graft Substitutes industry is projected to reach a value of USD 3.8 Billion by 2028, at a CAGR of 5.9% during the forecast period 2022-2028
Vantage Market Research is a collection of market research studies on several industries, such as Chemicals, semiconductors & Electronics, Food & Beverages Technology,…
Graft Delivery Devices Market Facts, Figures and Analytical Insights, 2017 to 20 …
Global Graft Delivery Devices Market: Snapshot
Graft delivery relates to the surgically transplantation of a piece of living tissues and the procedure can be highly essential as well as complex at the same time. Consequently, technological advancements have paved way to graft delivery devices that can minimally breach bone, fat, and vascular parts of the body to transfer drugs during plastic and reconstructive surgeries as well as arthroscopic and orthopedic surgeries.…
Glass Graft Polyols Market 2019| BASF SE, Sinopec, Shell, Oltchim, The Dow Chemi …
Market Research Hub (MRH) has actively included a new research study titled “Global Graft Polyols Market” Research Report 2019 to its wide online repository. The concerned market is discoursed based on a variety of market influential factors such as drivers, opportunities and restraints. This study tends to inform the readers about the current as well as future market scenarios extending up to the period until forecast period limit; 2025. In…
Global Vascular Graft Market Growth Analysis Report 2018-2025Global Vascular Gra …
Research Report 2018-2025 on "Global Vascular Graft Market" which provides an outlook of current market value of Vascular Graft Market as well as the expected forecast of Rate on Investment (ROI) with growing CAGR of XX% in Vascular Graft Market by the end of 2025. The report on the global Vascular Graft market uses the top-down and bottom-up approaches to define, analyze, and describe the Vascular Graft market 2018 trends…
