Press release
Lipid Nanoparticles (LNPs) CDMO 2.0 Market Analysis, Opportunities & Forecast 2025-2034
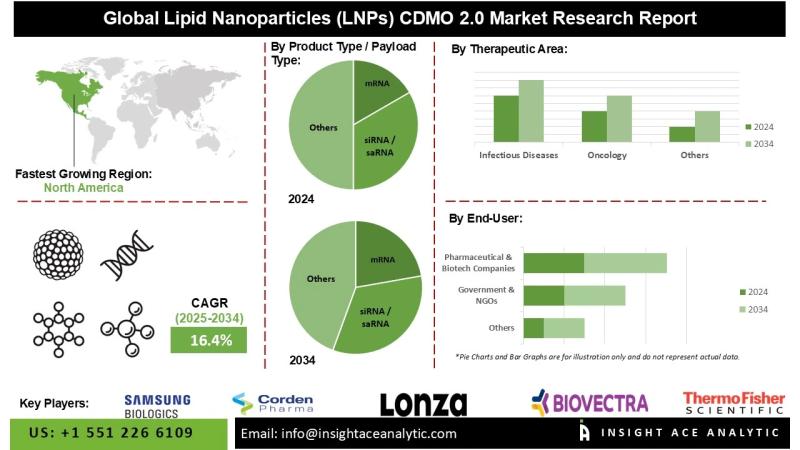
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Lipid Nanoparticles (LNPs) CDMO 2.0 Market"-, By Product Type / Payload Type (mRNA, siRNA / saRNA, Plasmid DNA (pDNA), CRISPR/Cas Components, microRNA, Antisense Oligos & Other Nucleic Acids), By Scale of Operation (Preclinical, Clinical (Phase I-III), Commercial), By CDMO 2.0 Business Model (Tech-Enabled CDMOs, Niche-Focused CDMOs, End-to-End Integrated CDMOs, Sustainability-Driven CDMOs), By Therapeutic Area (Infectious Diseases, Oncology, Rare & Genetic Disorders, Neurology & Regenerative Medicine), By End User (Pharmaceutical & Biotech Companies, Academic & Research Institutes, Government & NGOs), and Global Forecasts, 2025-2034 And Segment Revenue and Forecast To 2034."Lipid Nanoparticles (LNPs) CDMO 2.0 Market Size is predicted grow at a 16.4 % CAGR during the forecast period for 2025-2034.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/3184
The Lipid Nanoparticles (LNPs) CDMO 2.0 model is the next generation of contract development and manufacturing companies, providing sophisticated, comprehensive solutions for vaccines and treatments enabled by LNPs. With the development of next-generation ionizable lipids, PEG substitutes, and biodegradable LNPs, this model goes beyond the initial wave of LNP CDMOs created during the COVID-19 mRNA vaccine boom. It extends modalities beyond mRNA and siRNA to include saRNA, circular RNA, DNA, gene-editing payloads like CRISPR and TALENs, and even protein delivery.
From lipid synthesis and formulation to analytical characterization, scale-up, GMP production, and fill-finish, integrated services cover the whole value chain, guaranteeing smooth tech transfer for both start-ups and major pharmaceutical corporations. The foundation of CDMO 2.0 is smart manufacturing, which uses modular facilities that can quickly adapt to a variety of programs, continuous manufacturing for increased scalability and cost-efficiency, and AI/ML-driven LNP design to forecast stability and biodistribution.
The Lipid Nanoparticles (LNPs) CDMO 2.0 market is experiencing strong growth, fueled by the rise of advanced drug delivery technologies essential for complex therapeutics like nucleic acids and biologics. This surge aligns with the broader push toward precision medicine and the growing demand for rapid vaccine development and gene therapies, driving the need for scalable, stable, and compliant manufacturing solutions. Innovations in lipid chemistry, increased biotech-CDMO collaborations, and the shift to end-to-end service models are enhancing efficiency from formulation to commercialization.
Looking ahead, the LNPs CDMO 2.0 market presents several promising opportunities. Integrating sustainable practices such as incorporating eco-friendly lipids and minimizing production waste can appeal to environmentally conscious stakeholders while offering potential cost advantages. The shift toward regionalized supply chains is also projected to witness opportunities to reduce global disruptions and improve access to advanced therapies in emerging markets, where healthcare demand is on the rise.
List of Prominent Players in the Lipid Nanoparticles (LNPs) CDMO 2.0 Market:
• CordenPharma
• Lonza Group AG
• Thermo Fisher Scientific (Patheon)
• Evonik Health Care (Vancouver site - formerly Transferra Nanosciences)
• WuXi Biologics / WuXi AppTec
• BIOVECTRA
• Catalent
• Samsung Biologics
• Rentschler ATMP
• Curapath
• eTheRNA Manufacturing
• Phosphorex
• NOF CORPORATION
• NeoSome Life Sciences
• Helix Biotech
• NanoImaging Services (NIS)
• Envol Biomedical
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2025-04
Market Dynamics:
Drivers:
The market for lipid nanoparticles (LNPs) CDMO is expanding rapidly due to developments in drug delivery methods and the emerging field of mRNA therapies. The growing cooperation between biotechnology companies and contract development and manufacturing organizations (CDMOs) to improve the efficiency and scalability of LNP production is a significant trend. This partnership is essential to addressing the growing need for cutting-edge treatments.
The increasing investment in R&D to optimize LNP formulations for targeted drug delivery, especially in oncology and uncommon disorders, is another noteworthy trend. Innovation in LNP technology, with an emphasis on enhancing bioavailability and minimizing adverse effects, is being propelled by the drive for customized medicine. Additionally, regulatory developments are crucial since they promote the use of LNP-based treatments through expedited approval procedures.
Challenges:
The market for lipid nanoparticles (LNPs) CDMO faces a number of important obstacles and difficulties. The complex and expensive production procedures needed for LNPs are a major obstacle that may discourage new competitors and restrict scalability. Another challenge is regulatory barriers, since it can be costly and time-consuming to navigate the complicated approval process for innovative drug delivery systems.
Another issue facing the market is a lack of qualified workers, which makes it difficult to satisfy the rising demand for services linked to LNP. As businesses negotiate patent protections and any infringements, intellectual property issues further complicate the situation and may result in expensive legal disputes.
Regional Trends:
North America has the largest market share during the forecast period. North America faces a high burden of chronic diseases like cancer, diabetes, and cardiovascular disorders, which account for the largest global deaths per the World Health Organization. LNPs are ideal for targeted therapies. The region's focus on personalized medicine, supported by advanced healthcare infrastructure, increases the need for flexible, high-throughput CDMO platforms to produce tailored LNP-mRNA therapies.
However, the Asia Pacific is fastest fastest-growing region, which is becoming a key hub for lipid nanoparticle (LNP)-based medicines due to significant expenditures in biopharmaceutical manufacturing and healthcare infrastructure. The demand for novel, tailored treatments given via LNPs is fueled by the region's huge and diversified population's high burden of chronic diseases, including diabetes and cancer, as well as enduring infectious diseases.
Growth is also being accelerated by regulatory impetus; this was most evident in April 2024 when streamlined approval processes for LNP-based treatments were implemented, conforming to FDA and EMA guidelines. By cutting approval times by four to six months, these measures have improved the region's attractiveness to biotech entrepreneurs and encouraged more CDMO investments to satisfy domestic and international demand.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customization/3184
Recent Developments:
• In Jan 2025, Evonik collaborated with ST Pharm, a business that produces gene therapy active ingredients, to increase the scope of its RNA and nucleic acid therapeutic offerings. Through the collaboration, Evonik will be able to offer ST Pharm's tailored nucleic acids in addition to its range of lipid and lipid nanoparticle (LNP) therapeutic product development services. Pharmaceutical companies can accelerate the speed-to-market and decrease complexity for nucleic acid therapies by using this simplified technique. Evonik's Health Care business, which is a part of the company's Nutrition & Care division, is expanding its portfolio of system solutions for nucleic acid therapies by partnering with industry and life sciences leaders and making large investments.
• In June 2024, CordenPharma, a contract development and manufacturing company, said that it has joined forces with Certest, a Spanish business that specializes in API synthesis and drug delivery using lipid nanoparticles (LNPs), to create a line of ionizable lipids for LNP formulations. CordenPharma specializes in producing injectable drug products, LNPs comprising xRNA/xDNA, and drug substances for complicated modalities, including peptides, N-acetylgalactosamine (GalNAc), and lipids, among others.
Global Lipid Nanoparticles (LNPs) CDMO 2.0 Market- By Product Type / Payload Type
• mRNA
• siRNA / saRNA
• Plasmid DNA (pDNA)
• CRISPR/Cas Components
• microRNA, Antisense Oligos & Other Nucleic Acids
Global Lipid Nanoparticles (LNPs) CDMO 2.0 Market - By Scale of Operation
• Preclinical
• Clinical (Phase I-III)
• Commercial
Global Lipid Nanoparticles (LNPs) CDMO 2.0 Market - By CDMO 2.0 Business Model
• Tech-Enabled CDMOs
• Niche-Focused CDMOs
• End-to-End Integrated CDMOs
• Sustainability-Driven CDMOs
Global Lipid Nanoparticles (LNPs) CDMO 2.0 Market- By Therapeutic Area
• Infectious Diseases
• Oncology
• Rare & Genetic Disorders
• Neurology & Regenerative Medicine
Global Lipid Nanoparticles (LNPs) CDMO 2.0 Market - By End User
• Pharmaceutical & Biotech Companies
• Academic & Research Institutes
• Government & NGOs
Global Lipid Nanoparticles (LNPs) CDMO 2.0 Market - By Region
North America-
• The US
• Canada
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• Southeast Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Mexico
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of the Middle East and Africa
Read Overview Report- https://www.insightaceanalytic.com/report/lipid-nanoparticles-cdmo-market/3184
Why should buy this report:
To receive a comprehensive analysis of the prospects for the global Lipid Nanoparticles (LNPs) CDMO 2.0 Market. To receive an industry overview and future trends of the global Lipid Nanoparticles (LNPs) CDMO 2.0 Market
To analyze the Lipid Nanoparticles (LNPs) CDMO 2.0 Market drivers and challenges
To get information on the Lipid Nanoparticles (LNPs) CDMO 2.0 Market. size value (US$ Mn) forecast till 2034
Major Investments, Mergers & Acquisitions in the Cloud-Based and AI-Driven Eye Tracking Systems industry
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 607 400-7072
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Lipid Nanoparticles (LNPs) CDMO 2.0 Market Analysis, Opportunities & Forecast 2025-2034 here
News-ID: 4180629 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
Automotive Lead Acid Battery Market Strategic Growth Drivers and Outlook 2026 to …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Lead Acid Battery Market Size, Share & Trends Analysis Report By Product (SLI and Micro-Hybrid Batteries), Type (Flooded, Enhanced Flooded, and VRLA), Customer Segment (OEM and Aftermarket), End User (Passenger Car, Light Commercial Vehicles, Heavy Commercial Vehicles, Two-Wheeler, and Three-Wheeler), and Application (Hybrid Vehicles, Electric Vehicles, Light Motor Vehicles, and Heavy Motor Vehicles)- Market…
Automotive Interior Market Investment Opportunities and Forecast 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Interior Market- (By Component Type (Center Stack, Head-up Display, Instrument Cluster, Rear Sear Entertainment, Dome Module, Headliner, Seat, Interior Lighting Door Panel, Center Console, Adhesives & Tapes, Upholstery, Others), By Material (Leather, Fabric, Vinyl, Wood, Glass Fiber Composite, Carbon Fiber Composite, Metal), By Level of Autonomy (Semi-Autonomous, Autonomous, Non-Autonomous),By Electric Vehicle (Battery Electric Vehicle…
Artificial General Intelligence Market Future Landscape and Industry Evolution 2 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Artificial General Intelligence (AGI) Market - (By Type of Offering (Hardware, Software and Service), Type of Technology (Machine Learning, Deep Learning, Natural Language Processing and Robotics), Mode of Deployment (Cloud-based, On Premise and Web-based), Type of AI (Weak AI, Strong AI and Superintelligence), Type of Processing (Image, Text and Voice Processing), Company Size (SMEs and…
Allogenic Cell Therapies Market Revenue Trends and Growth Potential 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Allogenic Cell Therapies Market- by Cell Type(Cardiosphere-Derived Cells (CDCs), Fibroblasts, T-cells, Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs) and Others),Tissue Source(Skin, Blood, PBC, BM and Others), Indication (Acute graft-versus-host disease (GVHD), Chronic Ulcers and Diabetic Foot Ulcers, Osteoarthritis, Crohn's Disease, Cardiovascular Disease, Solid Tumors/Cancers and Others (Alzheimer's Disease, etc.)), Trends, Industry Competition Analysis, Revenue…
More Releases for LNP
Lipid Nanoparticles (LNP) Market Outlook and Future Projections for 2030
The lipid nanoparticles (lnp) market represents a dynamic and continually evolving landscape, shaped by changing consumer demands and technological advancements. In this comprehensive report, we provide an in-depth exploration of the market, designed for a wide range of stakeholders including manufacturers, suppliers, distributors, and investors. Our goal is to equip industry participants with essential insights that enable informed decision-making in an ever-changing market environment. This analysis not only examines the…
LNP-based Therapies: A Look at the High-Growth LNP CDMO Market Fueling Innovatio …
The Lipid Nanoparticles (LNPs) CDMO Market to reach over USD 518.2 Mn by the year 2031 - Exclusive Report by Insight Ace Analytic
"The Lipid Nanoparticles (LNPs) CDMO Market" in terms of revenue was estimated to be worth $182.0 Mn in 2023 and is poised to reach $518.2 Mn by 2031, growing at a CAGR of 14.10% from 2024 to 2031 according to a new report by Insight Ace Analytic.
Get…
CD Bioparticles Announces Comprehensive Assay Portfolio for mRNA-LNP Vaccine Dev …
CD Bioparticles is pleased to announce a suite of mRNA-LNP Vaccine Laboratory Process Development Assays.
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, is pleased to announce a suite of comprehensive mRNA-LNP Vaccine [https://www.cd-bioparticles.net/services/bioparticles-analysiscand-characterization/mrna-lnp-vaccine-laboratory-process-development-assay] Laboratory Process Development Assays. This latest addition to CD Bioparticles' extensive service portfolio is specifically designed for the rapid and efficient development of mRNA-LNP vaccines.
The mRNA molecule is well known to…
Lipid Nanoparticles (LNP) Market 2021, Current Trade Size, Country Level Analysi …
(United States, Portland): The latest research report added by Big Market Research on the Survey of Lipid Nanoparticles (LNP) Market is intended to offer reliable data on various key factors shaping the growth curve & outlook of Lipid Nanoparticles (LNP) market. This report works as a rich source of information for key entities such as policy makers, end-use industries, investors, and opinion leaders.
The Demand analysis of Lipid Nanoparticles (LNP) Market…
SABIC’s new LNP™ ELCRES™ EXL resin delivers superior flame retardance
The implementation of the International Electrotechnical Commission’s new IEC 62368-1 safety standard for consumer electronics is prompting many manufacturers to seek higher-performing flame-retardant (FR) materials. Realme, a leading Chinese smartphone manufacturer, has selected SABIC’s new LNP™ ELCRES™ EXL7414 copolymer resin for the battery enclosure of its C25 phone to achieve UL 94 V0 FR compliance at 0.6mm, addressing the new IEC standard. Additionally, the superior flame retardance of the new…
SABIC HOSTS FIRST EUROPEAN LNP™ ANNIVERSARY TECHNICAL SUMMIT
BERGEN OP ZOOM, THE NETHERLANDS, June 11, 2019 - SABIC is holding a series of technical summits around the world to mark 70 years of its LNP™ product line of engineering thermoplastic compounds and copolymers. Following a series of events in Asia that began late last year, SABIC has initiated a schedule of events in cities across Europe and the USA.
The European leg began in mid-May at SABIC’s facilities in…