Press release
LNP-based Therapies: A Look at the High-Growth LNP CDMO Market Fueling Innovation Building Better Drugs
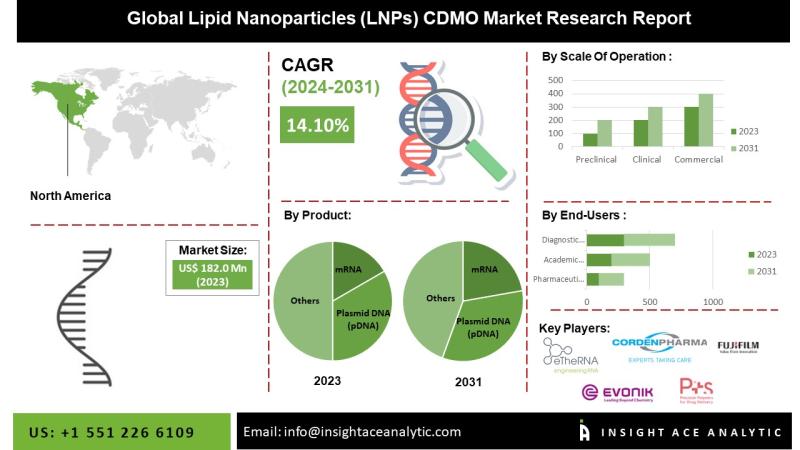
The Lipid Nanoparticles (LNPs) CDMO Market to reach over USD 518.2 Mn by the year 2031 - Exclusive Report by Insight Ace Analytic"The Lipid Nanoparticles (LNPs) CDMO Market" in terms of revenue was estimated to be worth $182.0 Mn in 2023 and is poised to reach $518.2 Mn by 2031, growing at a CAGR of 14.10% from 2024 to 2031 according to a new report by Insight Ace Analytic.
Get a free sample copy of the report: https://www.insightaceanalytic.com/request-sample/1432
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global Lipid Nanoparticles (LNPs) CDMO Market are:
• The rising chronic and infectious diseases
• Personalized medicines
• An increasing number of health-conscious people
The following are the primary obstacles to the Lipid Nanoparticles (LNPs) CDMO market's expansion:
• High-cost outsourced services
• Lack of specialized expertise in nanoparticles production
• Stringent rules and regulations
Future expansion opportunities for the global The Lipid Nanoparticles (LNPs) CDMO market include:
• Rising collaboration for lipid nanoparticle manufacturing
• Oncology applications
• Adoption of single-use technology
Market Analysis:
One of major driving factors of the Lipid Nanoparticles (LNPs) CDMO market is the surging medical applications of nanoparticles. An increase in investments by key players in lipid nanoparticles to develop promising drug therapies, rising cases of chronic diseases, an increasing aging population, and a growing number of health-conscious people also contribute to the market's growth.
List of Prominent Players in the Lipid Nanoparticles (LNPs) CDMO Market:
• Laboratorios Farmacéuticos Rovi, S.A.
• Samsung Biologics
• Thermo Fisher Scientific
• Sartorius AG (BIA Separations)
• AGC Biologics
• Hanmi Pharmaceutical
• BioCina Pty Ltd.
• Catalent, Inc.
• Genevant Sciences
• Lonza Group AG
• Rentschler Biopharma
• Nitto Denko Avecia
• Evonik
• Orden Pharma GmbH
• eTheRNA
• Polypeptide Therapeutic Solutions(PTS)
• FUJIFILM Corporation
• ST Pharm Co Ltd.
• Exalead (Merck KGaA)
• Avanti Polar Lipids, Inc. (Croda International Plc.
• Emergent CDMO
• Esco Aster Pte Ltd
• Ernal Biosciences
• Recipharm AB
• Phosphorex Inc.
• Polymun Scientific Immunbiologische Forschung GmbH
• BIOVECTRA
• Vernal Biosciences
• Helix Biotech
Lipid Nanoparticles (LNPs) CDMO Market Report Scope:
Report Attribute Specifications
Market size value in 2023 USD 182.0 Mn
Revenue forecast in 2031 USD 518.2 Mn
Growth rate CAGR CAGR of 14.10% from 2024 to 2031
Quantitative units Representation of revenue in US$ Million, and CAGR from 2024 to 2031
Historic Year 2019 to 2023
Forecast Year 2024-2031
Report coverage The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends
Segments covered By Product, Scale of Operation, End-User
Regional scope North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Recent Developments:
• In May 2022, ST Pharm launched its global mRNA Consignment Development and Manufacturing Organization (CDMO) business by signing a lipid supply agreement with Biotech in North America. Lipids play a crucial role in the production of mRNA-LNPs.
• In Mar 2022, eTheRNA Manufacturing introduced the novel LNP formulation development and production services. The new LNP service allows for precise delivery and personalized distribution of substances by utilizing eTheRNA's unique lipid libraries and formulations and also allows customers to optimize the delivery of their RNA products by leveraging the expertise of its specialized team.
• In July 2021, Curia (US), formerly AMRI, a contract research, development, & manufacturing organization, acquired Integrity Bio, Inc., a biopharmaceutical & fill-finish organization in Camarillo, to enhance its biologics drug product formulation development and fill-finish network.
Curious about this latest version of the report? @ https://www.insightaceanalytic.com/enquiry-before-buying/1432
The Lipid Nanoparticles (LNPs) CDMO Market Dynamics:
Market Drivers: Personalized Medicines
Personalized medicines are essential for delivering drugs at specifically targeted sites. Liposomes, niosomes, nanoparticles, and nanotechnology-based drug delivery systems are helpful in several therapeutic areas. It is projected to be effective in a subset of patients,
leaving others with either ineffective treatment or treatment that causes significant toxicity. Furthermore, Lipid nanoparticles are used as a carrier in delivering oncology and neurology-related drugs. It is anticipated to individualize/customize therapeutic management based on the patient's characteristics to overcome blanket treatment.
Challenges: Stringent Rules and Regulations
The stringent rules and regulations imposed on the industries to make and market drugs have restricted them from growing. The laws issued by several governments also restricted the inflow of R&D investments for developing complex drugs using nanoparticles.
Furthermore, stringent regulatory necessities for drug approval and safety testing pose essential barriers to Lipid Nanoparticles development and commercialization. Meeting regulatory values takes time and effort, restraining the growth of the industry.
North America Is Expected To Grow With the Highest CAGR during the Forecast Period
The North America the Lipid Nanoparticles (LNPs) CDMO Market is likely to register a significant revenue share and develop at a rapid CAGR soon. This is due to the increasing awareness about the applications of nanoparticles, increasing government interest and investments in research and developments.
The increasing prevalence of chronic diseases drives the North American market. Furthermore, the region is expected to dominate the market owing to the well-established healthcare facilities & CMOS, the rising demand for specialized drugs, and the increasing number of clinical trial advancements in the biopharmaceutical industries.
Segmentation of the Lipid Nanoparticles (LNPs) CDMO Market-
By Product-
• mRNA
• Plasmid DNA (pDNA)
• siRNA
• saRNA
• microRNA
• Others
By Scale of Operation
• Preclinical Scale Operations
• Clinical Scale Operations
• Commercial Scale Operations
By End-User-
• Pharmaceuticals Companies
• Academic Research Institutes
• Diagnostic Laboratories
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• South East Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/1432
Contact Us:
InsightAce Analytic Pvt. Ltd.
Tel.: +1 718 593 4405
Email: info@insightaceanalytic.com
Site Visit: www.insightaceanalytic.com
Follow Us on LinkedIn @ bit.ly/2tBXsgS
Follow Us On Facebook @ bit.ly/2H9jnDZ
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions.
Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses.
We help clients gain a competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets, and repositioning products.
Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release LNP-based Therapies: A Look at the High-Growth LNP CDMO Market Fueling Innovation Building Better Drugs here
News-ID: 3541950 • Views: …
More Releases from InsightAce Analytic Pvt.Ltd
AI In The Credit-Scoring Market Smarter Credit Decisions: How AI is Transforming …
AI In The Credit-Scoring Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global AI In The Credit-Scoring Market - (By Component (Software and Service), By Application (Personal Credit Scoring and Corporate Credit Scoring), By Industry Vertical (BFSI (Banking, Financial Services, Insurance), Retail, Healthcare,
Telecommunications, Utilities, and Real Estate)), Trends,…
Surgical Robotics Simulation Market Guiding the Next Generation of Surgeons: Gro …
Surgical Robotics Simulation Market Worth $1,283.6 Mn by 2031 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Surgical Robotics Simulation Market Size, Share & Trends Analysis Report By Product Type (Product, Services), By Application (General Surgery, Gynecological Surgery, Urological Surgery, Neurological Surgery (Head and Neck Surgery), Cardiological Surgery, Orthopedic Surgery, Others), By End User (Hospitals, Ambulatory Surgical Centers,…
AI Studio Market Accelerating Innovation: The Benefits of AI Studios for Busines …
Global AI Studio Market Worth $57.89 Bn by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global AI Studio Market- (By Application (Sentiment Analysis, Customer Service Automation, Image Classification & Labelling, Synthetic Data Generation, Predictive Modelling & Forecasting, Automatic Content Generation, and Others), By Offering, By Vertical, By Region, Trends, Industry Competition Analysis, Revenue and Forecast…
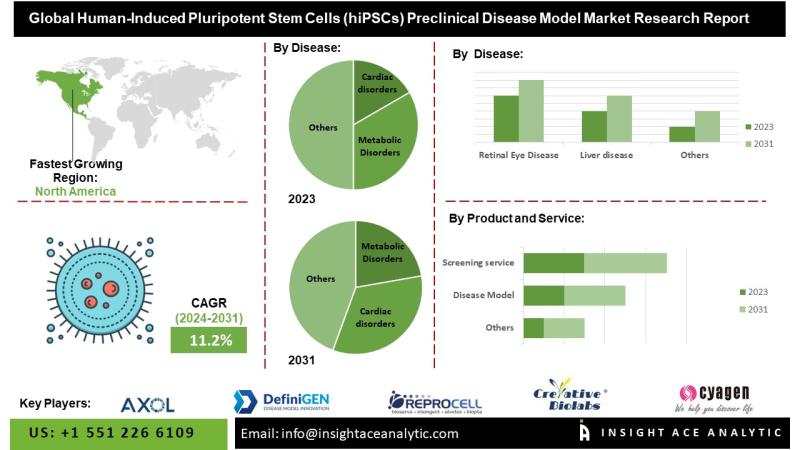
Human-Induced Pluripotent Stem Cells (hiPSCs) Preclinical Disease Model Market G …
Global Human-Induced Pluripotent Stem Cells (hiPSCs) Preclinical Disease Model Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Human-Induced Pluripotent Stem Cells (hiPSCs) Preclinical Disease Model Market- (Disease (Neurological Disorders and Dystrophies, Cardiac disorders, Retinal Eye Disease, Metabolic Disorders, Liver disease, Others), Products and Services (Disease Model,
Reprogramming service, Differentiation…
More Releases for LNP
Lipid Nanoparticles (LNP) Market Outlook and Future Projections for 2030
The lipid nanoparticles (lnp) market represents a dynamic and continually evolving landscape, shaped by changing consumer demands and technological advancements. In this comprehensive report, we provide an in-depth exploration of the market, designed for a wide range of stakeholders including manufacturers, suppliers, distributors, and investors. Our goal is to equip industry participants with essential insights that enable informed decision-making in an ever-changing market environment. This analysis not only examines the…
CD Bioparticles Announces Comprehensive Assay Portfolio for mRNA-LNP Vaccine Dev …
CD Bioparticles is pleased to announce a suite of mRNA-LNP Vaccine Laboratory Process Development Assays.
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, is pleased to announce a suite of comprehensive mRNA-LNP Vaccine [https://www.cd-bioparticles.net/services/bioparticles-analysiscand-characterization/mrna-lnp-vaccine-laboratory-process-development-assay] Laboratory Process Development Assays. This latest addition to CD Bioparticles' extensive service portfolio is specifically designed for the rapid and efficient development of mRNA-LNP vaccines.
The mRNA molecule is well known to…
Lipid Nanoparticles (LNP) Market 2021, Current Trade Size, Country Level Analysi …
(United States, Portland): The latest research report added by Big Market Research on the Survey of Lipid Nanoparticles (LNP) Market is intended to offer reliable data on various key factors shaping the growth curve & outlook of Lipid Nanoparticles (LNP) market. This report works as a rich source of information for key entities such as policy makers, end-use industries, investors, and opinion leaders.
The Demand analysis of Lipid Nanoparticles (LNP) Market…
SABIC’s new LNP™ ELCRES™ EXL resin delivers superior flame retardance
The implementation of the International Electrotechnical Commission’s new IEC 62368-1 safety standard for consumer electronics is prompting many manufacturers to seek higher-performing flame-retardant (FR) materials. Realme, a leading Chinese smartphone manufacturer, has selected SABIC’s new LNP™ ELCRES™ EXL7414 copolymer resin for the battery enclosure of its C25 phone to achieve UL 94 V0 FR compliance at 0.6mm, addressing the new IEC standard. Additionally, the superior flame retardance of the new…
SABIC HOSTS FIRST EUROPEAN LNP™ ANNIVERSARY TECHNICAL SUMMIT
BERGEN OP ZOOM, THE NETHERLANDS, June 11, 2019 - SABIC is holding a series of technical summits around the world to mark 70 years of its LNP™ product line of engineering thermoplastic compounds and copolymers. Following a series of events in Asia that began late last year, SABIC has initiated a schedule of events in cities across Europe and the USA.
The European leg began in mid-May at SABIC’s facilities in…
LNP AT 70: FULL OF ENERGY AND INNOVATION
AMSTERDAM, THE NETHERLANDS, November 9, 2018 - The LNP™ product line, part of global leader of diversified chemicals SABIC and the company’s foremost brand for engineering thermoplastics, this year celebrates 70 years of successful operation. A pioneer in compounding technologies, the LNP product brand has a string of important technological achievements behind it. “And scientists and technologists behind the LNP portfolio continue to push the limits of technical performance, solving…