Press release
AAV Vector-based Gene Therapy Market Poised to Growth USD 56.23 Billion by 2034 with Thriving CAGR of 25.68%
AAV Vector-based Gene Therapy Market: Unlocking Treatments for Genetic Diseases at ScaleMarket Overview
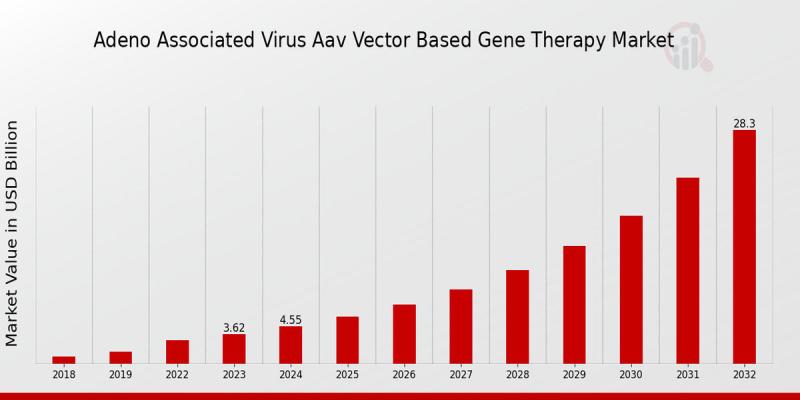
The AAV Vector-based Gene Therapy Market is on a steep growth trajectory as biotechnology firms, researchers, and healthcare systems converge on delivering safer, more effective treatments for genetic and rare diseases. As per MRFR analysis, the market was valued at USD 5.72 billion in 2024, is expected to grow to USD 7.19 billion in 2025, and is projected to reach USD 56.23 billion by 2034, with a powerful CAGR of about 25.68% over the 2025-2034 period.
This rise reflects not only growing prevalence of genetic disorders but also increasing regulatory approvals, vector engineering advances, and expansive clinical trial activity.
Why AAV-based Gene Therapies Are Gaining Ground
What makes AAV vector-based gene therapy especially promising in the current healthcare landscape is a combination of safety, flexibility, and expanding disease applicability. AAV vectors are less immunogenic than many viral vectors, and modern serotype engineering improves their ability to target specific tissues - whether for ophthalmic disease, neurological disorders, cardiovascular conditions, or rare genetic defects.
Researchers are increasingly pairing AAV delivery with advanced tools like CRISPR-Cas9 for more precise genome editing, as well as optimized vector design and promoter selection to maximize treatment efficacy. Patient advocacy groups and governments are strengthening funding and regulatory support, motivating biotech firms to push novel therapies through the pipeline. All these forces factor into the broader AAV Vector-based Gene Therapy Market analysis, which shows not just numeric growth, but qualitative shifts in how gene therapy is developed, delivered, and accessed.
Request To Free Sample of This Strategic Report -
https://www.marketresearchfuture.com/sample_request/29672
Key Players Driving Innovation
These are the companies leading the charge in developing, commercializing, or enabling AAV vector-based therapies:
uniQure
Lysogene
AveXis
Sangamo Therapeutics
Dimension Therapeutics
Regenxbio
Bluebird Bio
Audentes Therapeutics
Spark Therapeutics
Oxford BioMedica
Urogen Pharma
Solid Biosciences
ReNeuron
Voyager Therapeutics
Nightstar Therapeutics
These players are not only advancing gene therapy pipelines for diseases like spinal muscular atrophy, hemophilia, and inherited retinal disorders but are also investing in vector production scalability, better serotype design, regulatory interactions, and partnerships. Their activity is central to the AAV Vector-based Gene Therapy Market key manufacturers and competitive landscape.
🛒 Buy Now Premium Research Report -
https://www.marketresearchfuture.com/checkout?currency=one_user-USD&report_id=29672
Disease Types, Serotypes & Delivery Routes: Where Growth Is Strongest
Segment-wise, several trends stand out:
Disease Type: Oncology currently holds a large share (> 40%) due to cancer gene therapy trials, while neurological disorders, ophthalmic diseases, hematological malignancies, and cardiovascular diseases are expected to grow strongly.
Vector Serotypes: AAV serotypes like AAV8 and AAV9 are increasingly preferred due to broad tropism and lower immune rejection. Serotypes AAV1, AAV2, AAV5, AAV6 also remain important for specific tissues.
Administration Routes: Intravenous delivery is dominant for systemic applications. Intramuscular, subcutaneous, intraocular, and intracerebral routes are growing in use for localized delivery and organ-specific therapies.
This segmentation reveals key AAV Vector-based Gene Therapy Market segment dynamics, showing where R&D and commercial investment are most concentrated.
Browse In-depth Market Research Report (Pages, Charts, Tables, Figures)
https://www.marketresearchfuture.com/reports/adeno-associated-virus-aav-vector-based-gene-therapy-market-29672
Regional Trends: Dominance, Emerging Players, and Market Share
Geographically, North America remains the powerhouse for AAV vector-based gene therapy, supported by strong biotech ecosystems, regulatory clarity, and large patient bases. Europe follows with growing investment and adoption. Asia-Pacific is rapidly catching up with increasing government support, investment in gene therapy infrastructure, and clinical trial expansion. South America and the Middle East & Africa are newer entrants, gradually increasing their role as local manufacturing, awareness, and regulatory frameworks improve.
These regional patterns contribute to the AAV Vector-based Gene Therapy Market regional share, highlighting where growth potential is greatest and where access lags but opportunity exists.
Patient Experience, Clinical Impacts, and Real-World Outcomes
Behind the numbers are patients who stand to benefit: those with inherited retinal diseases, spinal muscular atrophy, hemophilia, or rare metabolic disorders. Early treatments using AAV vectors have already delivered life-changing outcomes in some cases - vision preservation, reduced disease burden, improved mobility.
Clinical trials are increasingly reporting long-term follow-up data indicating durable gene expression, which improves confidence in regulatory approval and adoption. Another key development: improved delivery and manufacturing reducing vector dose requirements, limiting immune reactions, and lowering cost. These shifts support both the AAV Vector-based Gene Therapy Market growth and improved patient access.
Technology & Innovation Trends to Watch
Some of the standout developments fueling future growth include:
Vector Engineering: Better capsid design for tissue targeting and immune evasion.
Gene Editing Synergies: Use of CRISPR and other technologies alongside AAVs for disease correction.
Manufacturing Scale-Up: Advances in production platforms, stable producer cell lines, purification methods, and CDMO capacity.
Regulatory & Safety Improvements: More robust safety profiling, fewer adverse events, and clearer regulatory paths.
These technology trends are central to the AAV Vector-based Gene Therapy Market developments and are key for sustaining momentum.
Outlook & Projections
With market projections showing growth from USD 7.19 billion in 2025 to about USD 56.23 billion by 2034, the forecast is aggressive. Key factors likely to influence this trajectory include regulatory approvals of more therapies, breakthroughs in delivery to challenging tissues such as the brain, scalable production, and policies enabling wider access (insurance, reimbursement, rare disease legislation).
What This Means for Stakeholders
Patients and clinicians can expect more available therapies, potentially for diseases that currently have little to no effective treatment.
Biotechs and developers will continue to focus on serotype optimization, vector dosing, and manufacturing efficiency.
Regulators and funders are likely to play increasing roles in shaping what's safe, efficacious, and affordable.
Payers and health systems will need to evaluate cost vs long-term benefit; durable gene therapy may require different reimbursement models.
Bottom Line
The AAV Vector-based Gene Therapy Market is no longer an emerging idea-it's becoming a mature growth engine of biotechnology. From its USD 5.72 billion base in 2024 to the projected USD 56.23 billion by 2034, it reflects not just economic expansion but also major advancements in science, patient care, and regulatory readiness. As innovation continues, the market's ability to deliver on the promise of treating genetic and rare diseases at scale will define its success.
Explore our latest reports
France Medical Aesthetics Market - https://www.marketresearchfuture.com/reports/france-medical-aesthetics-market-44969
Spain Medical Aesthetics Market - https://www.marketresearchfuture.com/reports/spain-medical-aesthetics-market-44975
UK Optical Coherence Tomography Market - https://www.marketresearchfuture.com/reports/uk-optical-coherence-tomography-market-44876
Japan Orthopedic Devices Market - https://www.marketresearchfuture.com/reports/japan-orthopedic-devices-market-44858
Germany Peripheral Nerve Stimulators Market - https://www.marketresearchfuture.com/reports/germany-peripheral-nerve-stimulators-market-44905
US Peripheral Nerve Stimulators Market - https://www.marketresearchfuture.com/reports/us-peripheral-nerve-stimulators-market-12836
Germany Robot-Assisted Surgical Systems Market - https://www.marketresearchfuture.com/reports/germany-robot-assisted-surgical-systems-market-45583
Japan Robot Assisted Surgical Systems Market - https://www.marketresearchfuture.com/reports/japan-robot-assisted-surgical-systems-market-45584
Spain Robot Assisted Surgical Systems Market - https://www.marketresearchfuture.com/reports/spain-robot-assisted-surgical-systems-market-45591
Germany Smart Healthcare Market - https://www.marketresearchfuture.com/reports/germany-smart-healthcare-market-45555
About Market Research Future:
Market Research Future (MRFR) is a global market research company that takes pride in its services, offering a complete and accurate analysis with regard to diverse markets and consumers worldwide. Market Research Future has the distinguished objective of providing the optimal quality research and granular research to clients. Our market research studies by products, services, technologies, applications, end users, and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help answer your most important questions.
Contact:
Market Research Future (Part of Wantstats Research and Media Private Limited)
99 Hudson Street, 5Th Floor
New York, NY 10013
United States of America
+1 628 258 0071 (US)
+44 2035 002 764 (UK)
Email: sales@marketresearchfuture.com
Website: https://www.marketresearchfuture.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release AAV Vector-based Gene Therapy Market Poised to Growth USD 56.23 Billion by 2034 with Thriving CAGR of 25.68% here
News-ID: 4179394 • Views: …
More Releases from Market Research Future

Digitally Printed Wallpaper Market Size estimated at USD 6.5 Billion in 2024 and …
The digitally printed wallpaper market is experiencing a significant transformation, driven by advancements in technology, changing consumer preferences, and a growing emphasis on sustainability. As of 2024, the market was valued at approximately $6.5 billion, and it is projected to grow to $40.9 billion by 2035, with a compound annual growth rate (CAGR) of 18.2% from 2025 to 2035. This article explores the key trends, drivers, and future outlook of…

Glue Laminated Timber Market outlook shows steady growth from USD 3.508 Billion …
The construction industry is undergoing a significant transformation, driven by the increasing demand for sustainable building materials. Among these materials, glue laminated timber (glulam) has emerged as a frontrunner, offering a combination of strength, versatility, and environmental benefits. The Glue Laminated Timber Market is projected to grow from $3.41 billion in 2024 to $4.661 billion by 2035, with a compound annual growth rate (CAGR) of 2.88% during the forecast period…

Fiber Cement Board Market Size and future outlook project industry expansion fro …
The fiber cement board market is experiencing significant growth, driven by increasing demand for sustainable building materials and advancements in construction technologies. This article explores the key trends, market dynamics, and future outlook for the fiber cement board industry.
Overview of the Fiber Cement Board Market
According to the latest analysis by Market Research Future, the fiber cement board market was valued at approximately USD 14446.52 million in 2024 and is projected…

Plastic Pipes Industry Market Size highlights consistent growth from USD 26.25 B …
The plastic pipes market is experiencing significant growth, driven by various factors including urbanization, sustainability initiatives, and technological advancements. As of 2024, the market size is estimated at $26.25 billion, with projections indicating it could reach $44.9 billion by 2035, growing at a compound annual growth rate (CAGR) of 5.0% from 2025 to 2035. This article explores the key trends, drivers, and future outlook of the plastic pipes market.
Get a…
More Releases for AAV
AAV Gene Therapy: $5.72B to $39.45B | 21.3% CAGR
Why are AAV vectors considered one of the safest and most efficient gene delivery systems?
Adeno-associated virus (AAV) vectors have gained prominence as one of the most reliable, safe, and clinically effective viral delivery platforms in the gene therapy landscape. Their favorable safety profile, ability to deliver therapeutic genes with precision, and long-term expression capabilities make them ideal for addressing rare diseases, inherited conditions, and chronic disorders.
One of the core reasons…
AAV Vector Transfection Kits Market Key Players, Share and Forecast Outlook
"The global market for AAV (Adeno-Associated Virus) vector transfection kits is poised for significant growth, currently valued at approximately $1.2 billion in 2024. This market is projected to reach around $3 billion by 2034, reflecting a robust compound annual growth rate (CAGR) of 9.5% during the forecast period of 2025-2034. "
Exactitude Consultancy., Ltd. released a research report titled "AAV Vector Transfection Kits Market". This report covers the global AAV Vector…
ProBio offers AAV One-stop Solution for AAV vector
AAV One-stop Solution
Process development for triple transfection
Support regulatory filing
AAV vector is widely used delivery vehicle due to its high safety and effectiveness in delivering Gene of Interest (GOI). ProBio is broadening its business in AAV services [https://www.probiocdmo.com/gct-one-stop-aav.html]to cater to the market demand.
Image: https://www.probiocdmo.com/img/probio/gct-one-stop-aav-banner.jpg
One-stop Solution for AAV
ProBio offers services from cell banking, process development, AAV packaging [https://www.probiocdmo.com/gct-one-stop-aav.html], analytical development, to GMP manufacturing and stability test for AAV vector. ProBio is also…
AAV Contract Development And Manufacturing Organizations Market 2024 Insights an …
In recent years, the global AAV Contract Development And Manufacturing Organizations Market has witnessed a dynamic shift, influenced by changing consumer preferences, technological advancements, and a growing emphasis on sustainability. The Research report on AAV Contract Development And Manufacturing Organizations Market presents a complete judgment of the market through strategic insights on future trends, growth factors, supplier landscape, demand landscape, Y-o-Y growth rate, CAGR, pricing analysis. It also provides and…
Adeno-Associated Virus (AAV) Vectors in Gene Therapy Pipeline Outlook Report 202 …
DelveInsight has released its latest report titled "AAV Vectors in Gene Therapy Pipeline Insight 2024" offering extensive insights into over 70 companies and more than 235 pipeline drugs within the AAV vectors gene therapy landscape. This comprehensive report includes detailed profiles of pipeline drugs across clinical and nonclinical stages, alongside thorough assessments based on product type, development stage, route of administration, and molecule type. Additionally, it features an analysis of…
Adeno-Associated Virus (AAV) CDMO Services Market Opportunities and Forecast 202 …
Data Library Research newly added a research report on the Adeno-Associated Virus (AAV) CDMO Services Market, which represents a study for the period from 2022 to 2029. The research study provides a near look at the market scenario and dynamics impacting its growth. This report highlights the crucial developments along with other events happening in the market which are marking on the growth and opening doors for future growth in…