Press release
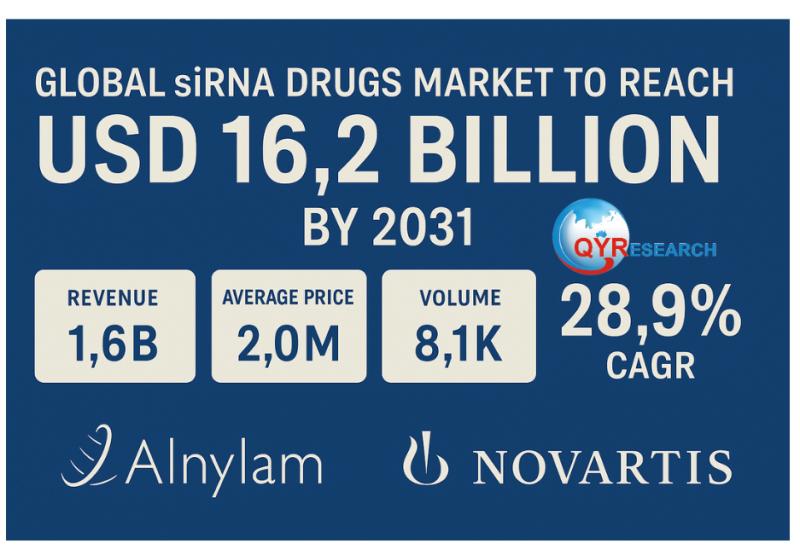
Global siRNA Drugs Market to Reach USD 16.2 Billion by 2031 with 28.9% CAGR Driven by Alnylam and Novartis
According to the recent report from QYResearch, RNAi therapeutics are moving rapidly from promise to practice. Anchored by breakthroughs in cardiovascular disease and transthyretin amyloidosis, small interfering RNA (siRNA) drugs are reshaping global treatment strategies. Recent clinical trial readouts, regulatory approvals, and commercial performance in 2024-2025 have positioned the sector for strong growth, with global revenues projected to reach US$16.2 billion by 2031 at a 28.9% CAGR.Latest Data
• Forecasted market size (2031): US$ 16,238 million
• CAGR (2025-2031): 28.9%
• Regional split: USA ≈57% of global consumption, Europe second
• By product type: Subcutaneous injection >77% share, intravenous remainder
• By application: Nervous system therapy ≈52% share, followed by endocrine & metabolic, and others
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) https://www.qyresearch.com/sample/4771742
Leading Companies
Alnylam
Novartis
Novo Nordisk
Genzyme
Arrowhead
Silence
Sylentis
Avidity Biosciences
Sirnaomics
Classification
• Intravenous Injection
• Subcutaneous Injection
Applications
• Nervous System Therapy
• Endocrine and Metabolic Therapy
• Others
2024-2025 Highlights
Alnylam - AMVUTTRA (vutrisiran): In 2025, Alnylam expanded vutrisiran into ATTR-CM with approvals in Europe, UK, Japan, and Brazil. Long-term data showed a 36% reduction in all-cause mortality and 33% reduction in cardiovascular mortality through 42 months, reinforcing the drug's role beyond biomarkers. Quarterly dosing supports adoption and sales in Q2 2025 reached $492 million, with around 1,400 ATTR-CM patients treated.
Novartis - LEQVIO (inclisiran): In July 2025, the FDA approved inclisiran as a first-line monotherapy for LDL-C reduction, broadening its use beyond add-on therapy. Inclisiran sales in 2024 reached $754 million (up 112% year-on-year). The twice-yearly subcutaneous dosing model has gained traction across the US and Europe, including NHS-supported rollout in England.
Arrowhead - plozasiran (ARO-APOC3): Phase 3 PALISADE results in 2024 demonstrated up to 80% median triglyceride reductions and significant decreases in acute pancreatitis incidence. This program targets familial chylomicronemia syndrome and severe hypertriglyceridemia, creating a foundation for expansion into larger dyslipidemia populations.
Silence Therapeutics - zerlasiran (SLN360): Mid-stage trial results in 2024 showed ~80-90% reductions in Lp(a) sustained up to 60 weeks with dosing intervals as long as 16-24 weeks. This positions zerlasiran among leading siRNA approaches in lipoprotein(a) lowering, addressing a major unmet cardiovascular risk factor.
Avidity Biosciences - del-desiran (AOC 1001): First-in-class muscle-targeted siRNA showed durable improvements in strength, myotonia, and daily functioning in myotonic dystrophy type 1 patients. The FDA lifted a partial clinical hold in late 2024, clearing the path for Phase 3 studies in 2025. This represents one of the first successful extrahepatic delivery programs for siRNA.
Product Snapshots
Alnylam - AMVUTTRA (vutrisiran)
• Target: TTR silencing
• Dosing: 25 mg subcutaneous every 3 months
• 42-month outcomes: 36% fewer deaths, 33% lower cardiovascular mortality
• Sales Q2 2025: $492M; ~1,400 ATTR-CM patients treated globally
Novartis - LEQVIO (inclisiran)
• Target: PCSK9 silencing
• Dosing: Initial, 3 months, then every 6 months
• LDL-C reduction: ~50% sustained long term (>6 years data)
• 2024 sales: $754M (+112% YoY); FDA first-line approval July 2025
Arrowhead - plozasiran (ARO-APOC3)
• Target: APOC3 silencing
• Phase 3: ~80% triglyceride reduction; reduced pancreatitis incidence
• Dosing: infrequent subcutaneous injections under study
Silence Therapeutics - zerlasiran (SLN360)
• Target: LPA silencing
• Phase 2: ~80-90% Lp(a) reduction; effect maintained up to 60 weeks
• Dosing: q16-24 weeks
Avidity Biosciences - del-desiran (AOC 1001)
• Target: DMPK silencing in muscle
• Data: Strength, myotonia, daily living improvements sustained long term
• Regulatory: FDA hold lifted 2024; Phase 3 initiated 2025
Market Trend
Subcutaneous Delivery Becomes Dominant
With more than 77% of global siRNA drug sales tied to subcutaneous formats, quarterly or semi-annual dosing has become standard. Inclisiran and vutrisiran prove that low-frequency injections significantly improve adherence and long-term outcomes, accelerating payer acceptance.
Cardiometabolic Expansion
Cardiovascular and metabolic diseases are set to become the largest drivers of siRNA adoption. Inclisiran's broad LDL-C lowering, plozasiran's impact on triglycerides, and zerlasiran's durable Lp(a) reductions signal that siRNA will expand far beyond rare diseases into large-scale public health markets.
Extrahepatic Delivery Breakthroughs
Historically restricted to the liver, siRNA is now successfully reaching muscle tissue with Avidity's AOC platform. This unlocks therapeutic opportunities in neuromuscular disorders and points toward possible future applications in the brain and other organs.
Hard Outcomes Strengthen Value
HELIOS-B results with vutrisiran delivered mortality and cardiovascular event reductions, elevating siRNA beyond surrogate biomarker evidence. Similar outcomes trials are underway in LDL-C and Lp(a) lowering, potentially cementing siRNA's role in guideline-directed care.
Policy and Regulatory Acceleration
The FDA's first-line approval for inclisiran in 2025 and NHS England's nationwide rollout provide strong regulatory tailwinds. These moves embed siRNA into primary care settings, ensuring earlier adoption across broader patient populations.
Manufacturing Scale and CDMO Growth
Global contract manufacturers have scaled siRNA output from grams to kilograms annually. Agilent, WuXi STA, and ST Pharm have all expanded capacity in 2024-2025 to support surging demand, driving down cost per dose and ensuring supply stability.
Next-Generation Delivery Chemistry
Advances in GalNAc design and chemical modifications now allow longer intervals between doses-16 to 24 weeks or more-while improving safety and reducing off-target effects. This trend underpins payer confidence and expands use in chronic disease management.
Request for Pre-Order Enquiry On This Report https://www.qyresearch.com/customize/4771742
Downstream Customers
Cleveland Clinic
Mayo Clinic
Mass General Brigham
Kaiser Permanente
Mount Sinai Health System
UCLA Health
NYU Langone Health
Stanford Health Care
UCSF Health
Houston Methodist
Intermountain Health
Geisinger
Ochsner Health
Cedars-Sinai
HCA Healthcare
Conclusion
The siRNA drugs sector has entered a new era in 2025, driven by landmark regulatory wins, strong commercial momentum, and robust clinical outcomes. With subcutaneous formats dominating, cardiometabolic expansion accelerating, and extrahepatic delivery maturing, siRNA therapies are on track to become the third great drug class after small molecules and antibodies. Industry leaders such as Alnylam, Novartis, Arrowhead, Silence, and Avidity are defining the competitive landscape, while hospitals and health systems worldwide prepare to integrate these therapies into standard care.
Chapter Outline:
Chapter 1: Introduces the report scope of the report, executive summary of different market segments (by region, product type, application, etc), including the market size of each market segment, future development potential, and so on. It offers a high-level view of the current state of the market and its likely evolution in the short to mid-term, and long term.
Chapter 2: key insights, key emerging trends, etc.
Chapter 3: Manufacturers competitive analysis, detailed analysis of the product manufacturers competitive landscape, price, sales and revenue market share, latest development plan, merger, and acquisition information, etc.
Chapter 4: Provides profiles of key players, introducing the basic situation of the main companies in the market in detail, including product sales, revenue, price, gross margin, product introduction, recent development, etc.
Chapter 5 & 6: Sales, revenue of the product in regional level and country level. It provides a quantitative analysis of the market size and development potential of each region and its main countries and introduces the market development, future development prospects, market space, and market size of each country in the world.
Chapter 7: Provides the analysis of various market segments by Type, covering the market size and development potential of each market segment, to help readers find the blue ocean market in different market segments.
Chapter 8: Provides the analysis of various market segments by Application, covering the market size and development potential of each market segment, to help readers find the blue ocean market in different downstream markets.
Chapter 9: Analysis of industrial chain, including the upstream and downstream of the industry.
Chapter 10: The main points and conclusions of the report.
Contact Details
Tel: +1 626 2952 442 ; +41 765899438(Tel & Whatsapp); +86-1082945717
Email: john@qyresearch.com; global@qyresearch.com
Website: www.qyresearch.com
About us:
QY Research has established close partnerships with over 71,000 global leading players. With more than 20,000 industry experts worldwide, we maintain a strong global network to efficiently gather insights and raw data.
Our 36-step verification system ensures the reliability and quality of our data. With over 2 million reports, we have become the world's largest market report vendor. Our global database spans more than 2,000 sources and covers data from most countries, including import and export details.
We have partners in over 160 countries, providing comprehensive coverage of both sales and research networks. A 90% client return rate and long-term cooperation with key partners demonstrate the high level of service and quality QY Research delivers.
More than 30 IPOs and over 5,000 global media outlets and major corporations have used our data, solidifying QY Research as a global leader in data supply. We are committed to delivering services that exceed both client and societal expectations.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global siRNA Drugs Market to Reach USD 16.2 Billion by 2031 with 28.9% CAGR Driven by Alnylam and Novartis here
News-ID: 4178176 • Views: …
More Releases from QYResearch Europe

Global Aerospace Grade Smart Assembly Lines Market 2024 USD 4251 Million to 2031 …
According to recent report from QYResearch, the global market for aerospace-grade smart assembly lines stood at US$4,251 million in 2024 and is projected to reach US$8,712 million by 2031 at a 10.2% CAGR (2025-2031). In 2024, approximately 670 lines were produced globally at an average selling price (ASP) of about US$6.343 million per line. These highly automated systems integrate AI, industrial robotics, advanced sensing, and digital control to deliver repeatable,…
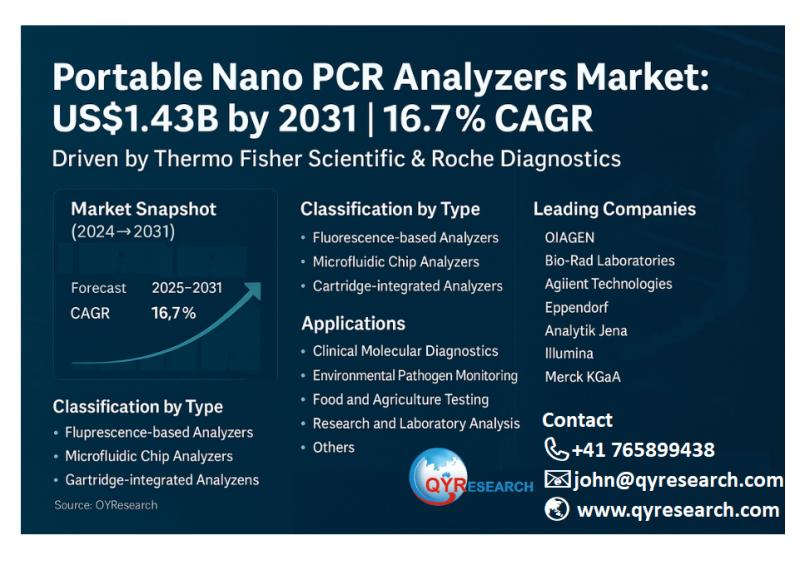
Portable Nano PCR Analyzers Market Growth to US$1.43 Billion by 2031 with 16.7% …
According to the latest QYResearch Report, the global market for Portable Nano PCR Analyzers was valued at US$484 million in 2024 and is expected to reach US$1,427 million by 2031, growing at a CAGR of 16.7% during the forecast period of 2025-2031. Global production in 2024 reached around 96,800 units, with an average price of about US$5,000 per unit. These portable devices utilize nanotechnology-enhanced PCR processes for rapid on-site genetic…

Global Multiphase Flow Conveying Equipment Market to Reach USD 10.88 Billion by …
The global market for Multiphase Flow Conveying Equipment is transitioning from a specialized engineering niche to a core enabler of industrial efficiency across upstream energy, chemicals, mining, and wastewater sectors. According to QYResearch 2025 edition of Multiphase Flow Conveying Equipment - Global Market Share and Ranking, Overall Sales and Demand Forecast 2025-2031, the market was valued at US$7,380 million in 2024 and is projected to reach US$10,879 million by 2031,…
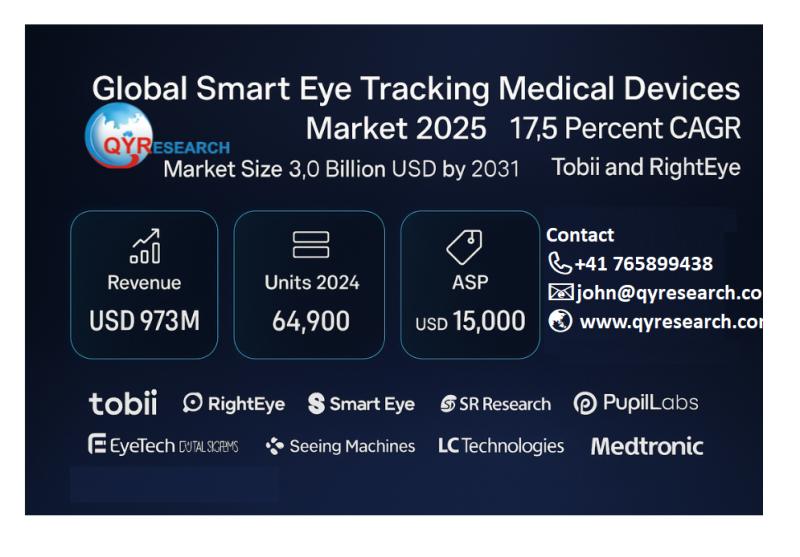
Global Smart Eye-Tracking Medical Devices Market Size Reaches US$3.0 Billion by …
The global Smart Eye-Tracking Medical Devices market has entered a stage of accelerated clinical adoption and product diversification. According to QYResearch 2025 Global Smart Eye-Tracking Medical Devices Market Research Report, the market was valued at US$973 million in 2024 and is projected to reach US$3,009 million by 2031, growing at a CAGR of 17.5% from 2025 to 2031. Global output in 2024 reached approximately 64,900 units, with an average price…
More Releases for RNA
CD Formulation Launches Custom Circular RNA Synthesis Service to Accelerate RNA …
CD Formulation introduces a customizable circRNA synthesis service, delivering high-quality, stable circRNAs for therapeutics, vaccines, and gene research, supported by advanced design and QC processes.
CD Formulation, a leading provider of advanced small nucleic acid synthesis [https://www.formulationbio.com/nucleic-acid/custom-small-nucleic-acid-synthesis.html] solutions, is proud to announce the launch of its fully customizable circular RNA (circRNA) synthesis service. This new service addresses the growing need for stable, non-immunogenic RNA molecules for therapeutic development, vaccine research, and…
Self-Amplifying RNA Synthesis Market Gains Traction as Biotech Firms Embrace Sca …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " Self-Amplifying RNA Synthesis Market- (By Product & Service (Products (Enzymes & Reagents, Premade saRNA, Others), Custom Synthesis Services), By Application (Therapeutics Development (Oncology, Infectious Diseases, Others), Biomedical Research), By End-User (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic,…
RNA Extraction and RNA Purification Market: Growth, Trends & Competitive Landsca …
The global RNA Extraction and RNA Purification Market is expected to grow at 6.3% CAGR from 2025 to 2032.
This Market Report is the result of extensive research and analysis conducted by our team of experienced market researchers through -
• 70% efforts of Primary Research
• 15% efforts of Secondary Research
• 15% efforts from the subscription to Paid database providing industry overview, macro and micro economics factors, and financials of private limited…
RNA Targeting Small Molecules Therapeutics Market: Exponential Growth with Risin …
Estimations Predict a CAGR of 29.8% by 2029 in Global RNA Targeting Small Molecules Therapeutics Market Boosted by Precision Medicine, RNA Biomarker Identification and RNA Genetic Manipulation
What Is The Projected Market Size of The Global RNA Targeting Small Molecules Therapeutics Market And Its Growth Rate?
• The market will grow from $6.1 billion in 2024 to $7.87 billion in 2025 at a compound annual growth rate (CAGR) of 28.9%.
• Expected exponential…
Global DNARNA Extraction Kit Market by Type (Cell-free DNA (cfDNA), Sequence-spe …
"DNARNA Extraction Kit Market" is segmented by Company, Region (country), By Type, Application, stakeholders and other participants. This report provides an analysis of revenue and forecast across Type and Application segments for 2023-2032.
The market for DNARNA Extraction Kits has been thoroughly researched via primary and secondary sources to produce this research study. Along with a competitive analysis of the market, segmented by application, type, and geographical trends, it offers a…
Cancer RNA Expression Market to Reap Excessive Revenues by 2028(By sequencing te …
Worldwide cancer is one of the leading cause of death and effective way of treating it still looks unaccomplished in most parts of the world. The factors which influence the successful treatment of cancer are different depending on the stage of diagnosis, treatment availability and availability of trained healthcare professionals coupled with high economic burden of the disease. The gene expression of cancerous cells varies by cancer type and may…