Press release
Global Biodegradable Implants Market Grows at 8.9% CAGR, Fueled by Orthopedic Demand & Biomaterial Advancements
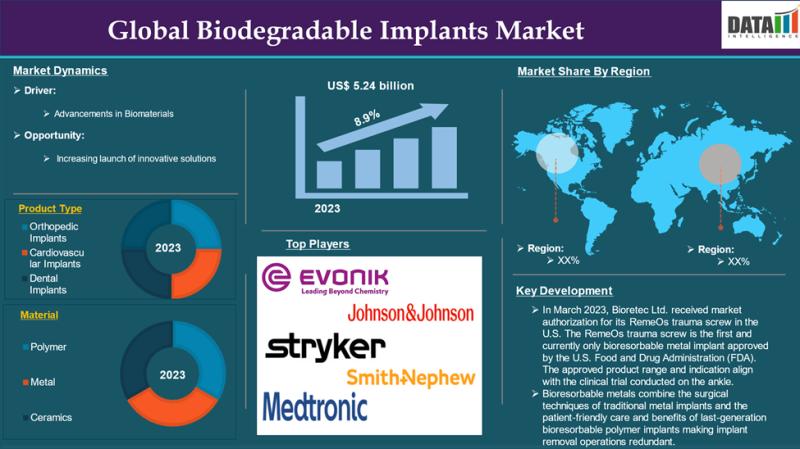
The global biodegradable implants market was valued at US$ 5.24 billion in 2023 and is projected to reach US$ 10.31 billion by 2031, growing at a compound annual growth rate (CAGR) of 8.9% during the forecast period 2024-2031.Market Overview
Biodegradable implants are medical devices designed to provide temporary support to damaged or diseased tissues, particularly in orthopedic, cardiovascular, and dental applications. Made from biocompatible materials like polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactone (PCL), these implants degrade naturally in the body, eliminating the need for surgical removal. They support healing while minimizing complications like infection or implant loosening. The market is driven by advancements in biomaterials, rising prevalence of chronic conditions, and increasing demand for minimally invasive solutions.
📌 Access valuable insights with our sample report - (Please enter your Corporate Email ID to get priority access):- https://www.datamintelligence.com/download-sample/biodegradable-implants-market?rk
Market Opportunities
Key opportunities include expanding applications in drug delivery systems, tissue engineering, and oncology, as well as growing adoption in emerging markets with improving healthcare infrastructure. Innovations in sustainable, biocompatible materials and regulatory approvals further enhance market potential.
Key Trends
Advancements in biodegradable polymers for improved biocompatibility and performance.
Increasing use of bioabsorbable implants in minimally invasive surgeries.
Growing focus on sustainable medical solutions aligning with environmental concerns.
Recent Developments
March 2023: Bioretec Ltd. received FDA approval for its RemeOs trauma screw, the first bioresorbable metal implant for ankle fractures.
March 2023: Invibio Biomaterial Solutions launched PEEK-OPTIMA AM Filament, an implantable PEEK polymer optimized for additive manufacturing.
July 2021: Stryker received FDA clearance for a balloon implant for arthroscopic treatment of massive irreparable rotator cuff tears.
March 2021: Inion received FDA approval for the CompressOn bioabsorbable compression screw for orthopedic applications.
June 2021: Researchers at Northwestern and George Washington Universities developed a wireless, battery-free, fully implantable transient pacemaker that degrades after use.
Market Dynamics: Drivers
Advancements in Biomaterials
Innovations in biodegradable materials, such as PLA, PGA, and PCL, are key drivers, enhancing implant safety and efficacy. These materials reduce risks of rejection and inflammation, making them ideal for temporary support in orthopedics and cardiovascular applications. For instance, Bioretec's RemeOs trauma screw, the first FDA-approved bioresorbable metal implant (March 2023), and Invibio's PEEK-OPTIMA AM Filament for additive manufacturing (March 2023) highlight advancements driving market growth. Regulatory approvals from the FDA and EMA further encourage investment and adoption.
Market Dynamics: Restraints
Regulatory Challenges
Stringent regulatory requirements, such as the FDA's extensive testing protocols and Europe's Medical Device Regulation (MDR), pose challenges. Lengthy approval timelines and rigorous clinical evaluations increase costs and delay market entry for new biodegradable implants, potentially deterring innovation and investment, particularly for smaller manufacturers.
📌 Speak to Our Senior Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/biodegradable-implants-market
Market Segment Analysis
By Product Type: Orthopedic Implants Dominate
The orthopedic implants segment holds a significant market share in 2024, driven by rising bone-related conditions like osteoporosis and fractures, particularly among the aging population. Globally, 37 million fragility fractures occur annually in individuals over 55, with osteoporosis affecting 21.2% of women and 6.3% of men over 50 (National Institute of Health). Biodegradable screws, pins, and plates, such as Inion's CompressOn screw (FDA-approved, March 2021), reduce the need for follow-up surgeries, enhancing patient outcomes.
By Material
Polymers like PLA, PGA, and PCL dominate due to their biocompatibility and ability to mimic natural bone properties, supporting tissue regeneration.
By Application
Orthopedic applications lead, followed by cardiovascular and dental, with growing use in drug delivery and tissue engineering.
By End-User
Hospitals and surgical centers are primary users, driven by high procedure volumes, followed by clinics and research institutes.
By Region
North America dominates, while Asia-Pacific is the fastest-growing region due to increasing healthcare investments and chronic disease prevalence.
Geographical Share
North America Holds Significant Share
North America accounted for a dominant market share in 2024, driven by advanced medical infrastructure and high chronic disease prevalence, including 3.4 million annual ankle fractures and 10.55 million adults with atrial fibrillation in the U.S. (National Institute of Health, 2024). Innovations like Stryker's FDA-cleared balloon implant for rotator cuff tears (July 2021) and a robust R&D ecosystem solidify the region's leadership.
Asia-Pacific Grows Rapidly
Asia-Pacific is the fastest-growing region, fueled by rising healthcare investments and an aging population. Osteoporosis affects 10-30% of women over 40 in the region, with 500-1000 fractures per 100,000 person-years among those over 50 (National Institute of Health, 2023). Countries like China, India, and Japan are modernizing healthcare facilities, increasing demand for biodegradable implants.
📌 Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=biodegradable-implants-market
Major Players
Key companies in the biodegradable implants market include:
Evonik
Johnson & Johnson
Medtronic
Stryker
Smith+Nephew
Boston Scientific Corporation
Inion
Bioretec
Arthrex, Inc.
Syntellix
📌 Request for 2 Days FREE Trial Access: https://www.datamintelligence.com/reports-subscription
☛ Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
☛ Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Biodegradable Implants Market Grows at 8.9% CAGR, Fueled by Orthopedic Demand & Biomaterial Advancements here
News-ID: 4171956 • Views: …
More Releases from DataM Intelligence 4market Research LLP

AI in Logistics Market Set to Reach $306.76B by 2032, Growing at 42% CAGR, Led b …
The AI in Logistics Market reached US$ 15.28 billion in 2024 and is expected to grow to around US$ 306.76 billion by 2032, expanding with a CAGR of approximately 42 % from 2025 to 2032 as businesses adopt intelligent technologies to drive efficiency and resilience in logistics operations.
Growth is supported by increasing demand across key applications such as predictive analytics for demand forecasting, route optimization, autonomous vehicles & drones, robotic…
Optometry Equipment Market to Reach USD 6.1B by 2031 | 6.2% CAGR Driven by OCT & …
Optometry Equipment Market Size and Forecast
The Global Optometry Equipment Market reached USD 3.8 billion in 2022 and is projected to witness lucrative growth by reaching up to USD 6.1 billion by 2031. The Global Optometry Equipment Market is expected to exhibit a CAGR of 6.2% during the forecast period 2024-2031.
DataM Intelligence has published a new research report on "Optometry Equipment Market Size 2025". The report explores comprehensive and insightful…
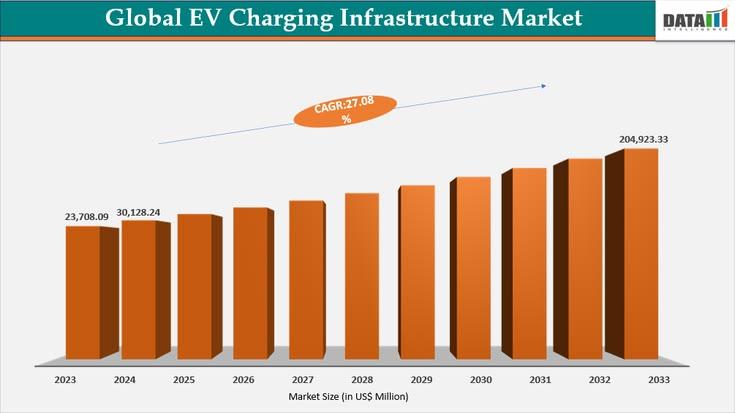
EV Charging Infrastructure Market to Reach US$ 204.9 Billion by 2032 Driven by N …
The EV charging infrastructure market reached a value of US$ 30,128.24 million in 2024, and is projected to reach US$ 204,923.33 million by 2032, growing at a robust CAGR of 27.08% during the forecast period 2025-2032.
Growth is driven by the rapid adoption of electric vehicles, supportive government policies, rising investments in public and private charging networks, and advancements in fast charging technologies. Increasing demand for residential, commercial, and public charging…
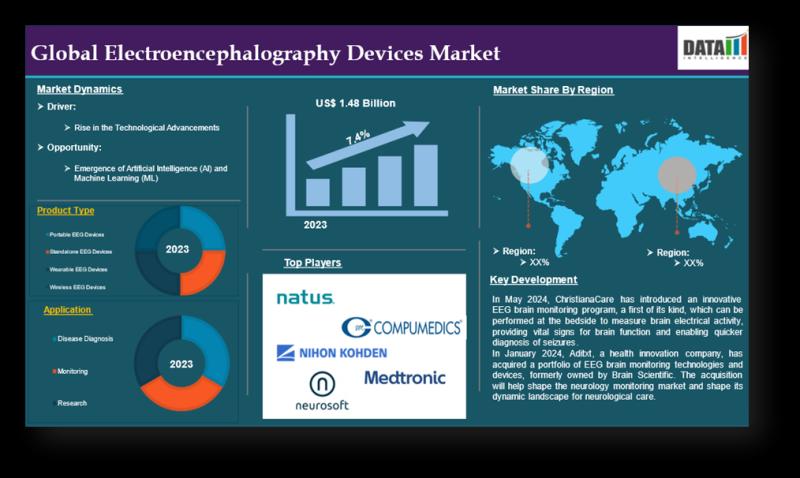
Electroencephalography Devices Market to Reach US$3.08B by 2033 | 7.4% CAGR Driv …
Electroencephalography Devices Market Size and Forecast
Electroencephalography Devices Market reached US$ 1.48 billion in 2023 and is expected to reach US$ 3.08 billion by 2033, growing at a CAGR of 7.4% during the forecast period 2025-2033.
DataM Intelligence has published a new research report on "Electroencephalography Devices Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…