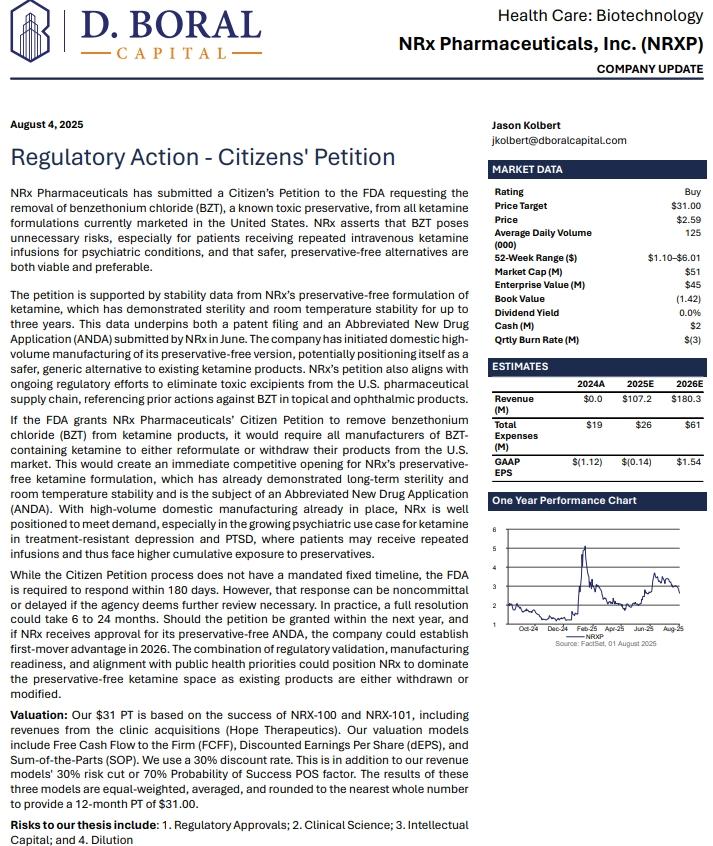
Press release
Advancements on Multiple Fronts in $3 Billion Suicidal Depression Market, Highlighted by FDA Fast Track Designation for Effective NRX 100 Drug Therapy: NRx Pharmaceuticals, Inc. (Nasdaq: NRXP)
Image: https://www.globalnewslines.com/uploads/2025/08/1755525181.jpg$NRXP Sees 10-Fold Market Expansion to 13 Million Americans for Bipolar Depression Alone.
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
* Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need, Based on FDA's Assessment of Data Submitted.
* 13 Million Adults Seriously Consider Suicide Each Year, According to the CDC, 3.2 Million Make a Plan to Commit Suicide.
* Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA).
* Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
* $7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with Universal Capital, LLC.
* Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents. The Company believes that its current cash position will support operations into 2026 and provide sufficient capital to reach expected regulatory inflection points.
The latest NRXP key developments included the following points:
NRx Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Completion of a toxicology assessment of Benzethonium Chloride1, documenting its lack of "Generally Recognized as Safe" (GRAS) status and lack of safety data to support its use in intravenous presentations of ketamine.
NRXP filing of a Citizen's Petition with the U.S. Food and Drug Administration to seek the removal of benzethonium chloride, a toxic preservative, from all ketamine products for intravenous administration.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
HOPE Therapeutics
NRXP execution of definitive Purchase Agreement and receipt of final regulatory clearance from Florida's Agency for Health Care Administration ("ACHA") to proceed with closing the acquisition of Dura Medical.
Execution of binding letter of intent to acquire the assets of NeuroSpa TMS Holdings of Tampa, FL.
Execution of a binding letter of intent to acquire a 49% interest in Cohen and Associates, LLC.
NRXP Receipt of approval, pending legal stipulations, for $7.8 million of debt financing to support the acquisition of Dura Medical, NeuroSpa TMS Holdings, and Cohen and Associates, LLC.
Execution of a definitive purchase agreement, subject to standard closing conditions and agreement between the parties regarding the resolution of ongoing discussions, to purchase the non-clinical assets of Kadima Neuropsychiatry Institute.
Execution of a non-binding term sheet for a strategic investment from a global medical device manufacturer into HOPE.
Corporate (subsequent to the filing of form 10-Q)
NRXP $6.5 million dollar investment to purchase approximately 3.9 million shares of common stock of NRx Pharmaceuticals on August 18, 2025, by a consortium of experienced biotechnology investors led by B Group Capital. The purchase is subject to a one-year lockup on trading, shorting, or otherwise hypothecating said securities. The investment has no warrants, repricing provisions, commissions, or other structure.
The B Group Capital led consortium of ultra long-term healthcare specialist investors is highly strategic with extensive experience in complex clinical, regulatory, and commercial therapeutics but also direct ownership and management of multi-unit retail operations with potentially positive long-term implications for efforts to continue to scale and develop NRXP HOPE Therapeutics.
For more information on $NRXP visit: https://www.nrxpharma.com/ [about:blank] and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/ [https://compasslivemedia.com/case-study/nrx-pharmaceuticals/]
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Media Contact
Company Name: NRx Pharmaceuticals, Inc.
Contact Person: Matthew Duffy, Chief Business Officer
Email: Send Email [http://www.universalpressrelease.com/?pr=advancements-on-multiple-fronts-in-3-billion-suicidal-depression-market-highlighted-by-fda-fast-track-designation-for-effective-nrx-100-drug-therapy-nrx-pharmaceuticals-inc-nasdaq-nrxp]
Phone: 484 254 6134
Address:1201 Orange Street Suite 600
City: Miami
State: Florida
Country: United States
Website: https://www.nrxpharma.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. GetNews makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Advancements on Multiple Fronts in $3 Billion Suicidal Depression Market, Highlighted by FDA Fast Track Designation for Effective NRX 100 Drug Therapy: NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) here
News-ID: 4148655 • Views: …
More Releases from Getnews

Remodel Republic Announces Home Remodeling Initiative for Georgia Homeowners
Remodel Republic has announced a Georgia-wide remodeling initiative offering complimentary kitchen and bathroom renovation estimates along with a $1,000 project incentive for qualifying homeowners. The initiative supports residents across metro Atlanta, including Alpharetta, Roswell, Milton, Marietta, Johns Creek, and Sandy Springs, as demand for kitchen and bathroom renovations continues to grow.
Atlanta, Georgia - February 18, 2026 - Remodel Republic, a Georgia-based kitchen and bathroom remodeling company, has announced a new…

How much is it to rent a yacht in 2026? A complete pricing guide
Image: https://www.globalnewslines.com/uploads/2026/02/1771399408.jpg
Understanding how much it costs to rent a yacht [https://boatstersblack.com/faq/how-much-does-it-cost-to-charter-a-yacht/] in 2026 requires analysing several interconnected factors. Boatsters Black [https://boatstersblack.com/], specialized in luxury yachts for charter across leading international destinations, highlights that pricing depends on vessel size, cruising area, seasonality and onboard service level. As demand for high-end maritime experiences continues to grow, clarity around cost structures has become increasingly relevant for clients planning premium travel.
In 2026, weekly yacht…

Baby Chiropractor Costa Mesa Shares Seasonal Advice for Managing Pregnancy Disco …
Image: https://www.globalnewslines.com/uploads/2026/02/1771393525.jpg
People tend to move more in spring, which may strain the pelvis and lower spine during pregnancy. The Webster Technique at our clinic helps restore the pelvis's balance, enabling the body to adjust to growth and weight changes. As tension around the uterus reduces, many mothers experience reduced nerve irritation and improved mobility as well as increased comfort.
Blooming Chiropractic in Costa Mesa, California specializes in prenatal, pediatric, family, and…

Bryant AR Roofer Launches Lifetime Metal Roofing Program to Combat Severe Storm …
Image: https://www.globalnewslines.com/uploads/2026/02/1771385166.jpg
Lifetime Construction Builders Introduces Hail-Resistant Metal Systems with Labor & Material Warranty for Saline County
As storm intensity increases across Central Arkansas, homeowners searching for a Bryant AR roofer are increasingly turning to metal roofing solutions for durability. In response to this demand, Lifetime Construction Builders has launched a specialized Metal Roofing Division in Bryant, offering high-performance standing seam and metal shingle systems designed to withstand the region's notorious hail…
More Releases for NRX
FDA Meeting Indicates Path to New Drug Application with Real World Data and Broa …
Image: https://www.globalnewslines.com/uploads/2026/02/1771336073.jpg
$NRXP Also Entered Joint Offering with and neurocare Group for Neuroplastic Therapy Targeting Depression, PTSD and Other Mental Health Afflictions
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* D. Boral Analyst Report on NRXP $34 Price Target.
* Type C Meeting with the FDA Demonstrates…
$40 Price Target in New H. C. Wainright Analyst Report on Leader in $3 Billion S …
Image: https://www.globalnewslines.com/uploads/2025/09/1757392285.jpg
$NRXP Continues Expansion with Completion of Dura Medical Acquisition in Network of Interventional Psychiatry Clinics
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression With Suicidality; Assuming Coverage…
$3 Billion Suicidal Depression Market May Soon be Accessible via FDA Fast Track …
Image: https://www.globalnewslines.com/uploads/2025/08/1754917343.jpg
$NRXP Has $7.8 Million for Clinic Acquisitions and Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* FDA Fast Track Designation for NRX…
$300 Million in Milestones Plus Tiered Double-Digit Royalties from Accepted Term …
Image: https://www.globalnewslines.com/uploads/2025/03/1742217317.jpg
$NRXP is Poised to Address Over $3 Billion Suicidal Depression Market in the US
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* New Drug Application for…
NRx Pharmaceuticals Highlights Breakthrough Oral Antidepressant's Efficacy in Re …
- Developing Therapeutics for the Treatment of CNS Disorders, Specifically Suicidal Bipolar Depression, Chronic Pain and PTSD.
- June Meeting of the American Society for Clinical Psychopharmacology Focused on Intravenous Ketamine and Intranasal S-Ketamine for Severe Depression and Suicidality.
- Presenters from 3 Open Label Studies at the ASCP Suggested Intravenous Ketamine is Equivalent or has Advantages over Intranasal S-Ketamine.
- NRXP Reached 9-Month Stability Point with its Ketamine Formulation (NRX-100) and Initiated…
Final Clinical Trial Results Show Superior Safety and Efficacy for NRX-101; Plan …
Developing Therapeutics for the Treatment of CNS Disorders, Specifically Suicidal Bipolar Depression, Chronic Pain and PTSD.
MOU Signed with Conversio Health with Immediate Plans to Ship IV Ketamine Product to Full Range of Customers via 503a and 503b Pharmacies.
Clinical Trial Success in Proving a Statistically-Significant 76% Reduction in Akathisia in Participants Treated with NRX-101 Compared to Lurasidone.
Company Plans to Seek Accelerated Approval of NRX-101 for Bipolar Depression and Akathisia and Broaden…