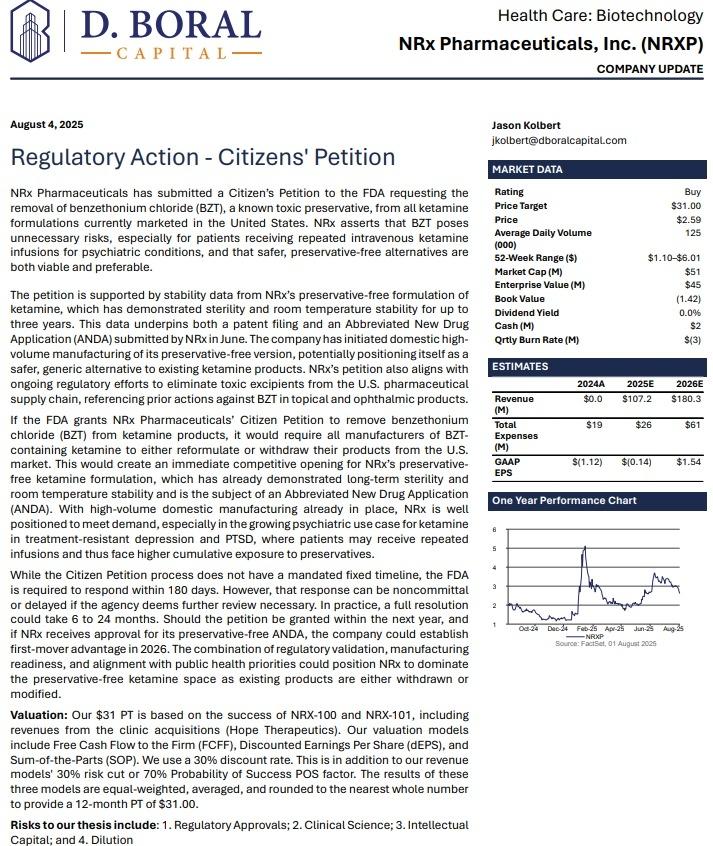
Press release
$3 Billion Suicidal Depression Market May Soon be Accessible via FDA Fast Track Designation for Effective NRX 100 Drug Therapy: NRx Pharmaceuticals, Inc. (Nasdaq: NRXP)
Image: https://www.globalnewslines.com/uploads/2025/08/1754917343.jpg$NRXP Has $7.8 Million for Clinic Acquisitions and Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
* Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need, Based on FDA's Assessment of Data Submitted.
* 13 Million Adults Seriously Consider Suicide Each Year, According to the CDC, 3.2 Million Make a Plan to Commit Suicide.
* Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA).
* Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
* $7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with Universal Capital, LLC.
* Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, including Bipolar Depression
On August 11th NRXP announced the US Food and Drug Administration (FDA) has granted Fast Track designation to its NRX-100 for the treatment of suicidal ideation in patients with depression, including bipolar depression. This designation for NRX-100 as a standalone drug is a 10-fold expansion of the addressable population for NRX-100, compared to the designation granted in 2017 for NRX-100 in combination with NRX-101 (DCS/lurasidone) for treatment of Suicidal Bipolar Depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet medical need, based on an assessment of the preliminary data contained in the Fast Track designation request. This determination of unmet medical need aligns with the eligibility requirements for the Commissioner's National Priority Voucher Program and for the FDA's Accelerated Approval Program.
NRXP has applied for a CNPV, which has the potential to substantially shorten the review cycle for NRX-100. Several well-controlled trials submitted to FDA in support of Fast Track Designation demonstrated a clinically meaningful and statistically significant reduction of suicidal ideation.
Regarding Fast Track designation, FDA's website states: A drug that receives Fast Track designation is eligible for some or all of the following:
More frequent meetings with FDA to discuss the drug's development plan and ensure collection of appropriate data needed to support drug approval.
More frequent written communication from FDA about such things as the design of the proposed clinical trials and use of biomarkers
Eligibility for Accelerated Approval and Priority Review, if relevant criteria are met.
Rolling Review, which means that a drug company can submit completed sections of its Biologic License Application (BLA) or New Drug Application (NDA) for review by FDA, rather than waiting until every section of the NDA is completed before the entire application can be reviewed. BLA or NDA review usually does not begin until the drug company has submitted the entire application to the FDA.
NRXP NRX-100 is poised to address the >$3 billion Suicidal Depression market in the US.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA)
On August 8th NRXP announced it has received final clearance and approval from the Florida Agency for Health Care Administration (AHCA) to proceed closing of its Dura Medical LLC acquisition, in connection with its change of ownership applications, a key regulatory step for closing. Dura is revenue generating and EBITDA positive.
Request to the Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products to Favor a Switch to Better Options
On August 4th NRXP announced its actions with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics and other products on the market today for safety concerns. The ketamine versions made by NRXP is BZT free and therefore presents a safer and superior option.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
For more information on $NRXP visit: https://www.nrxpharma.com/ [about:blank] and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Media Contact
Company Name: NRx Pharmaceuticals, Inc.
Contact Person: Matthew Duffy, Chief Business Officer
Email: Send Email [http://www.universalpressrelease.com/?pr=3-billion-suicidal-depression-market-may-soon-be-accessible-via-fda-fast-track-designation-for-effective-nrx-100-drug-therapy-nrx-pharmaceuticals-inc-nasdaq-nrxp]
Phone: 484 254 6134
Address:1201 Orange Street Suite 600
City: Miami
State: Florida
Country: United States
Website: https://www.nrxpharma.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. GetNews makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release $3 Billion Suicidal Depression Market May Soon be Accessible via FDA Fast Track Designation for Effective NRX 100 Drug Therapy: NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) here
News-ID: 4140698 • Views: …
More Releases from Getnews

Honghua Lang Illuminates Global Landmarks, Inviting the World to Share the Spiri …
As the Chinese New Year of the Horse arrives, a season defined by reunion, vitality and shared celebration unfolds around the world. From East to West, the Spring Festival continues to connect people through a collective sense of optimism, cultural pride and renewed beginnings.
Marking this auspicious moment, a distinguished expression of Chinese Red has illuminated iconic landmarks in major cities worldwide. Initiated by Honghua Lang, one of China's most esteemed…

SI Outdoor Elevates Colorado Landscapes Through Craftsmanship and Precision
Image: https://www.globalnewslines.com/uploads/2026/02/1770361591.jpg
Delivering refined outdoor environments across the Front Range through trust, technology, and timeless design
Arvada, CO - SI Outdoor [https://sioutdoor.com/] redefined what it means to be a modern outdoor contractor by delivering luxury landscapes built on structure, accountability, and enduring craftsmanship. Serving communities across Colorado's Front Range, SI Outdoor partners with homeowners, HOAs, and commercial clients to create outdoor environments that elevate properties and perform for the long term.
In 2025,…

Checking In on Atlanta: CAREGD Trademark Launches "Drop the Weight" to Normalize …
CAREGD launches Drop the Weight Atlanta, activating CARE-CHECK Trademark to embed emotional check-ins and crisis clarity into trusted Black community spaces.
Image: https://authoritypresswire.com/wp-content/uploads/2026/02/2026-Atlanta-Drop-the-Weight-CAREGD.png
ATLANTA, GA - In a city known for culture, faith, music, sports, and Black excellence, a new initiative is asking a simple but powerful question:
Are you good? Really?
CAREGDImage: https://s.w.org/images/core/emoji/15.0.3/72x72/2122.png is launching Drop the Weight Atlanta, to embed emotional check-ins and crisis clarity into trusted Black community spaces.
The movement was…

Apple Roofing Strengthens Local Reputation as Leading Leak Repair Roofer in Kear …
Image: https://www.globalnewslines.com/uploads/2026/02/1771427794.jpg
Kearney, NE - February 18, 2026 - Apple Roofing Kearney [https://appleroof.com/kearney/] continues to strengthen its reputation as a leading leak repair roofer in Central Nebraska, delivering professional roofing solutions backed by strong customer reviews, experienced craftsmanship, and a commitment to protecting homes and businesses from costly water damage.
As unpredictable Nebraska weather continues to challenge residential and commercial roofing systems, property owners throughout Kearney and surrounding communities are turning to…
More Releases for NRX
FDA Meeting Indicates Path to New Drug Application with Real World Data and Broa …
Image: https://www.globalnewslines.com/uploads/2026/02/1771336073.jpg
$NRXP Also Entered Joint Offering with and neurocare Group for Neuroplastic Therapy Targeting Depression, PTSD and Other Mental Health Afflictions
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* D. Boral Analyst Report on NRXP $34 Price Target.
* Type C Meeting with the FDA Demonstrates…
$40 Price Target in New H. C. Wainright Analyst Report on Leader in $3 Billion S …
Image: https://www.globalnewslines.com/uploads/2025/09/1757392285.jpg
$NRXP Continues Expansion with Completion of Dura Medical Acquisition in Network of Interventional Psychiatry Clinics
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression With Suicidality; Assuming Coverage…
Advancements on Multiple Fronts in $3 Billion Suicidal Depression Market, Highli …
Image: https://www.globalnewslines.com/uploads/2025/08/1755525181.jpg
$NRXP Sees 10-Fold Market Expansion to 13 Million Americans for Bipolar Depression Alone.
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
…
$300 Million in Milestones Plus Tiered Double-Digit Royalties from Accepted Term …
Image: https://www.globalnewslines.com/uploads/2025/03/1742217317.jpg
$NRXP is Poised to Address Over $3 Billion Suicidal Depression Market in the US
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* New Drug Application for…
NRx Pharmaceuticals Highlights Breakthrough Oral Antidepressant's Efficacy in Re …
- Developing Therapeutics for the Treatment of CNS Disorders, Specifically Suicidal Bipolar Depression, Chronic Pain and PTSD.
- June Meeting of the American Society for Clinical Psychopharmacology Focused on Intravenous Ketamine and Intranasal S-Ketamine for Severe Depression and Suicidality.
- Presenters from 3 Open Label Studies at the ASCP Suggested Intravenous Ketamine is Equivalent or has Advantages over Intranasal S-Ketamine.
- NRXP Reached 9-Month Stability Point with its Ketamine Formulation (NRX-100) and Initiated…
Final Clinical Trial Results Show Superior Safety and Efficacy for NRX-101; Plan …
Developing Therapeutics for the Treatment of CNS Disorders, Specifically Suicidal Bipolar Depression, Chronic Pain and PTSD.
MOU Signed with Conversio Health with Immediate Plans to Ship IV Ketamine Product to Full Range of Customers via 503a and 503b Pharmacies.
Clinical Trial Success in Proving a Statistically-Significant 76% Reduction in Akathisia in Participants Treated with NRX-101 Compared to Lurasidone.
Company Plans to Seek Accelerated Approval of NRX-101 for Bipolar Depression and Akathisia and Broaden…