Press release
US Animal Drug Compounding Market Expands with Veterinary Innovation - Persistence Market Research Report
The U.S. animal drug compounding market plays a pivotal role in the veterinary care industry, offering custom medications for pets, livestock, and exotic animals. Animal drug compounding refers to the process of modifying FDA-approved drug products under a veterinarian's prescription to meet the specific medical needs of an animal. These compounded drugs are typically used when an off-the-shelf FDA-approved product does not address the unique requirements of a particular animal. Unlike standard pharmaceutical products, compounded medications do not require FDA approval before use, which provides greater flexibility in treatment options.The market is experiencing steady growth due to increasing demand for customized treatments, unavailability of some branded drugs, and heightened awareness among pet owners about the importance of specialized care. Key drivers include a growing pet population, particularly in the U.S., advancements in veterinary medicine, and the increasing humanization of pets. According to Persistence Market Research, the rising adoption of pets and the expanding use of compounded drugs among veterinarians have catalyzed market growth.
✅Get a Sample Copy of Research Report (Use Corporate Mail id for Quick Response): https://www.persistencemarketresearch.com/samples/30058
Key Highlights from the Report
➤ The U.S. animal drug compounding market is primarily driven by the growing demand for customized medications for pets.
➤ The increasing unavailability of certain FDA-approved drugs is one of the major growth drivers for animal drug compounding.
➤ Compounded medications offer a solution for unmet therapeutic needs, especially for conditions like megacolon in cats.
➤ The growing awareness of the benefits of compounded medications among pet owners is increasing market demand.
➤ The market is benefiting from advancements in veterinary drug compounding technologies and improved drug formulations.
➤ Regional demand is highest in the U.S. due to the significant pet population and rising awareness regarding animal health.
Market Statistics and Growth Drivers
The U.S. animal drug compounding market has shown consistent growth over the past decade, driven by several key factors. One major growth driver is the unavailability of branded drugs with specific active ingredients required for treating certain conditions in animals. As discussed earlier, for example, the removal of drugs like Cisapride for treating megacolon in cats has led veterinarians to turn to compounded alternatives. This issue is not isolated to one medication but is widespread across various animal health conditions, propelling the demand for compounded treatments.
Another growth driver is the rise in the adoption of pets, especially dogs and cats, which has led to a greater need for specialized veterinary care. The increased awareness among pet owners about health conditions and treatments available for their animals has made them more likely to seek compounded solutions. According to industry reports, over 70 million dogs and 74 million cats were owned in the U.S. in 2015, and this number continues to rise. As pet owners become more informed, they increasingly seek out custom medications for their animals, further boosting market demand.
Leading Segment and Geographical Region
The most prominent segment in the animal drug compounding market is the companion animals segment, particularly dogs and cats. This is mainly because companion animals are more likely to suffer from chronic conditions that require tailored treatments. Additionally, the availability of flavored compounded medications has increased compliance among pet owners, particularly in pets with specific taste preferences. The livestock animal segment also plays a significant role, although it is comparatively smaller than the companion animal segment.
Geographically, the U.S. holds the dominant share of the animal drug compounding market. The reasons for this dominance include the high pet ownership rates, an advanced veterinary healthcare infrastructure, and a large number of compounding pharmacies. U.S. pet owners are more likely to invest in advanced veterinary care, which includes the use of compounded drugs for treating specific health issues. This trend is expected to continue throughout the forecast period, making the U.S. the leading market for animal drug compounding.
✅Read Detailed Analysis: https://www.persistencemarketresearch.com/market-research/us-animal-drug-compounding-market.asp
Market Segmentation
The U.S. animal drug compounding market is segmented based on various factors, including product type, animal type, and formulation type. These segments help to provide a clear understanding of market dynamics and offer insights into growth opportunities.
By Product Type:
The animal drug compounding market is categorized into CNS agents, anti-infective agents, hormones and substitutes, anti-inflammatory agents, and others. CNS agents and anti-inflammatory drugs are among the most commonly compounded medications due to the wide range of neurological and inflammatory conditions in animals that require specialized treatment options. Anti-infective agents also account for a substantial share, given the increasing prevalence of infections in both companion and livestock animals.
By Animal Type:
The market is divided into companion animals (dogs, cats, and others) and livestock animals. Companion animals, particularly dogs and cats, represent the largest portion of the market. The rising awareness among pet owners about the need for specialized care is fueling the demand for compounded drugs for these animals. Livestock animals also contribute to market growth, but the demand is less compared to companion animals, as livestock medication tends to follow a more standardized approach.
By Formulation:
Animal drug compounding formulations include oral, injectable, and other forms such as topical medications and transdermal patches. Oral formulations are the most commonly used in companion animals, given their ease of administration. However, injectables are widely used for more severe conditions that require rapid absorption or longer-lasting effects.
Regional Insights
In terms of regional trends, North America, particularly the United States, is leading the global market due to the high demand for veterinary care, an extensive pet population, and the increasing acceptance of compounded drugs. The U.S. is home to a large number of compounding pharmacies and a high level of regulatory oversight, which ensures a higher standard of quality in compounded medications. This region is expected to continue dominating the market during the forecast period.
Europe follows North America in terms of market share, with countries like the UK, Germany, and France contributing significantly to the growth of the market. The adoption of compounded drugs in veterinary care is also gaining traction in this region, especially in countries with a high level of pet ownership and an increasing awareness of animal health.
Market Drivers
The demand for animal drug compounding is primarily driven by several key factors. First, the unavailability of FDA-approved drugs for specific animal diseases, like megacolon in cats, creates a significant gap that can only be filled through compounded medications. Additionally, the rising pet population, especially in developed countries like the U.S., has led to an increased need for veterinary treatments that cater to specific medical conditions.
The humanization of pets is another driver of market growth. Pet owners now view their animals as family members, which has raised the expectations for their medical care. As a result, there is a growing demand for specialized and personalized veterinary treatments, including compounded medications that offer better outcomes for individual animals.
Market Restraints
Despite its growth, the animal drug compounding market faces several challenges. One major restraint is the lack of reimbursement for compounded drugs, which means that pet owners must cover the cost out-of-pocket. This can be a significant barrier to accessing compounded medications, especially in the case of chronic conditions that require ongoing treatment.
Another challenge is the regulatory landscape. While compounded medications offer greater flexibility, they are not subject to the same rigorous testing and approval processes as FDA-approved drugs. This raises concerns about the safety and efficacy of some compounded treatments, which could deter pet owners and veterinarians from using them.
✅Request for Customization of the Research Report: https://www.persistencemarketresearch.com/request-customization/30058
Market Opportunities
The increasing demand for flavored compounded medications presents a substantial opportunity for growth in the animal drug compounding market. Flavored formulations are particularly popular with pets that are picky eaters, such as cats, exotic pets, and even some zoo animals. This makes it easier for pet owners to administer medications and increases compliance, which is essential for effective treatment.
Moreover, the growing emphasis on compliance with regulatory standards and certifications like FDA and PCAB is likely to improve the overall quality and credibility of compounded medications. This could lead to higher consumer confidence and greater market acceptance in the long term.
Reasons to Buy the Report
✔ Comprehensive analysis of the U.S. animal drug compounding market, including key drivers, trends, and challenges.
✔ In-depth market segmentation and forecasting from 2019 to 2029 to aid in strategic decision-making.
✔ Insights into regional market dynamics and growth prospects in North America, Europe, and beyond.
✔ Detailed information on the key players operating in the market and their strategies.
✔ Analysis of regulatory factors and compliance trends affecting the animal drug compounding industry.
Company Insights
Some of the key players operating in the U.S. animal drug compounding market include:
✦ Hoye's Pharmacy
✦ Vertisis Custom Pharmacy
✦ Smith Caldwell Drug Store
✦ Sixth Avenue Medical Pharmacy
✦ Dougherty's Pharmacy
✦ Triangle Compounding Pharmacy Inc.
✦ Medisca Inc.
✦ Wedgewood Pharmacy
✦ Millers Pharmacy
Recent Developments:
FDA Approvals: Several U.S.-based animal drug compounding pharmacies have successfully passed FDA inspections, boosting consumer trust in compounded medications.
Flavored Compounded Drugs: A growing trend of offering flavored compounded medications for pets, such as tuna or sardine-flavored formulations for cats, has seen increased adoption in the market.
The U.S. animal drug compounding market is poised for significant growth, driven by increasing demand for personalized veterinary care, regulatory advancements, and the continued humanization of pets. As the market matures, both opportunities and challenges will shape its future trajectory.
Contact Us:
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release US Animal Drug Compounding Market Expands with Veterinary Innovation - Persistence Market Research Report here
News-ID: 4119464 • Views: …
More Releases from Persistence Market Research
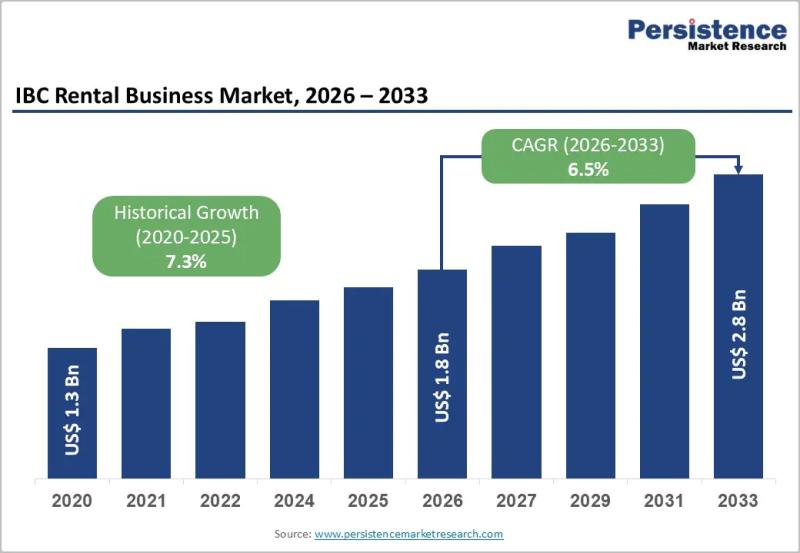
IBC Rental Business Market Size Expected to Reach US$2.8 Billion by 2033 - Persi …
The IBC rental business market has emerged as a critical component of modern industrial logistics and packaging ecosystems, driven by the growing need for flexible cost efficient and sustainable bulk liquid and semi liquid handling solutions. Intermediate Bulk Containers are widely used for transporting and storing chemicals food ingredients pharmaceuticals and industrial liquids due to their standardized design durability and safety advantages. Renting IBCs rather than owning them has gained…
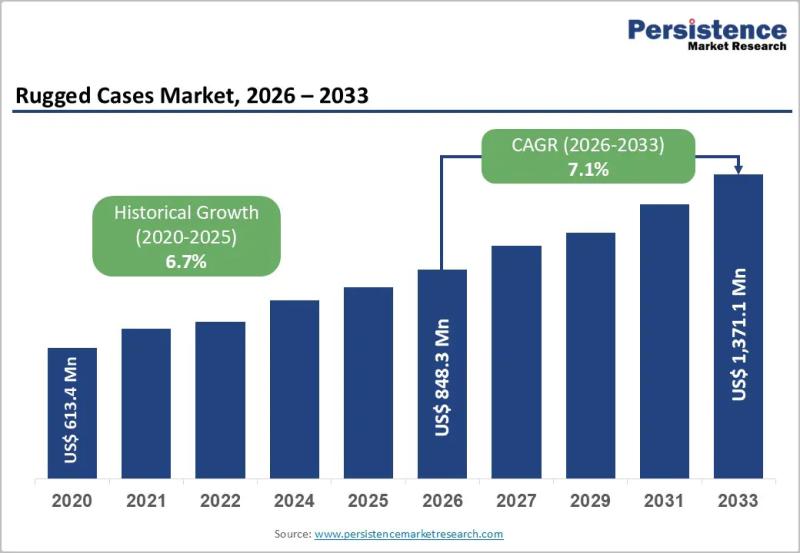
Rugged Cases Market Size to Reach US$1,371.1 Million by 2033 - Persistence Marke …
The rugged cases market has evolved into a critical segment within the broader protective packaging and equipment accessories industry, driven by the growing need to safeguard high value devices and sensitive equipment in harsh operating environments. Rugged cases are specifically engineered to provide superior protection against physical shocks vibration moisture dust and extreme temperatures. These cases are widely used across consumer electronics industrial equipment medical devices logistics military and government…
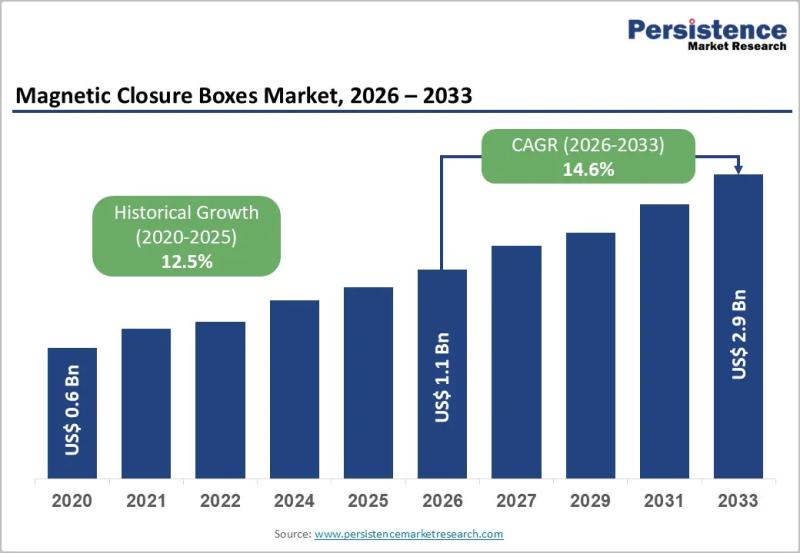
Magnetic Closure Boxes Market Forecast Shows Growth from US$1.1 Billion in 2026 …
The magnetic closure boxes market has emerged as a high value segment within the global rigid and premium packaging industry, driven by changing consumer expectations and brand strategies. Magnetic closure boxes are widely recognized for their premium aesthetics durability and reusability, making them a preferred packaging choice for luxury goods cosmetics electronics corporate gifting and high end retail products. These boxes combine rigid board construction with concealed magnetic mechanisms that…

Cold Chain Market to Reach US$ 919.9 Bn by 2032 as Key Players Lineage Logistics …
The cold chain market plays a critical role in maintaining the quality, safety, and efficacy of temperature-sensitive products across global supply chains. It encompasses a wide range of logistics solutions, including refrigerated storage, temperature-controlled transportation, monitoring systems, and handling infrastructure. Industries such as food and beverages, pharmaceuticals, biotechnology, and chemicals heavily rely on cold chain systems to prevent spoilage, contamination, and loss of product integrity. As global trade expands and…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
