Press release
A Smarter Way to Manage Batch Release Through APQR
Releasing a batch in pharma manufacturing isn't a small step; it's the final check before a product reaches patients. But if you're still relying on paper checklists, email follow-ups, and scattered approvals, the process can quickly become slow and error-prone.That's where integrating batch release into your APQR system makes a real difference.
Instead of working across disconnected tools, you can manage everything in one place. Start by setting up a Batch Release Readiness section in your APQR dashboard. This becomes your working checklist, showing exactly what has been done, what is pending, and what needs attention.
Each batch goes through a simple set of checks:
Manufacturing and packaging records are complete
- Quality control results are approved
- Any deviations are closed
- Stability samples are logged
- Final label checks are done
These aren't just boxes to tick. They're connected to the actual data, so nothing moves forward unless each step is complete. And to make it easier, use a colour system: green for done, yellow for pending, and red if something's missing. No more guesswork or digging through files.
When everything's in place, QA can sign off digitally. That means no chasing down signatures, no misplaced forms, and a clear record of who approved what and when.
Set up alerts too, so if something's overdue, the right people know. It's a small change that avoids last-minute surprises and helps batches move faster.
This approach not only replaces your process, but it also makes it tighter, clearer, and easier to manage. And it turns your APQR from a once-a-year task into a tool that actively supports your daily operations.
If you've been thinking about cleaning up your batch release workflow, this is a good place to start. Read here to know more - https://amplelogic.com/how-you-can-automate-batch-release-in-apqr/
AmpleLogic | GAMP Solutions | aPaaS for Life Sciences
- C Wing, 2nd Floor, Melange Tower, Huda Techno Enclave Rd, Patrika Nagar, HITEC City, Hyderabad, Telangana 500081
Press Contact - 7396660171
Email Address - marketing@amplelogic.com
AmpleLogic offers 14 SaaS products on a low-code platform built for life sciences. From APQR automation to batch records and document control, each solution is pre-configured for compliance and easy to customise, with no complex coding needed. The platform helps teams streamline quality processes, reduce manual work, and stay aligned with global standards like the US FDA and MHRA.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release A Smarter Way to Manage Batch Release Through APQR here
News-ID: 4104441 • Views: …
More Releases from AmpleLogic

AmpleLogic's AI Delivers 60% Manual Effort Reduction in Pharma Operations
Pharma companies using AmpleLogic's low-code platform are reporting significant improvements in day-to-day efficiency, with AI reducing manual effort by up to 60% across training, investigations, documentation, and APQR review work. The gains reflect a core challenge inside regulated operations: teams spend more time understanding information than executing workflows.
"Pharma teams aren't short of data; they're short of time," says Manne V. Chowdary, Founder & CEO of AmpleLogic. "Our focus has been…
AL Ideathon 2025 Launches to Ignite Innovation Across Industries
Mumbai, India - AmpleLogic, a pioneer in low-code platforms for regulated industries, has announced the launch of AL Ideathon 2025, an innovation challenge designed to empower students, professionals, and entrepreneurs to solve real-world industry problems with bold and practical ideas.
Driving Innovation
AL Ideathon has become one of India's most dynamic platforms for creative problem-solving, bringing together bright minds across pharmaceuticals, healthcare, sustainability, and digital transformation. For AmpleLogic, this initiative reflects its…

AL Ideathon 2.0 Opens for Registration: A New Season of Ideas Begins
AL Ideathon 2.0 is now open for registration. Scheduled for September 27, 2025, in Mumbai, the second edition of this industry-wide initiative is calling on pharma professionals to submit ideas that improve how work happens on the ground.
In its first season, AL Ideathon saw over 544+ ideas submitted by professionals working across production, QA, IT, and engineering functions. A total of ₹10,00,000 in prizes was awarded, a clear signal that…
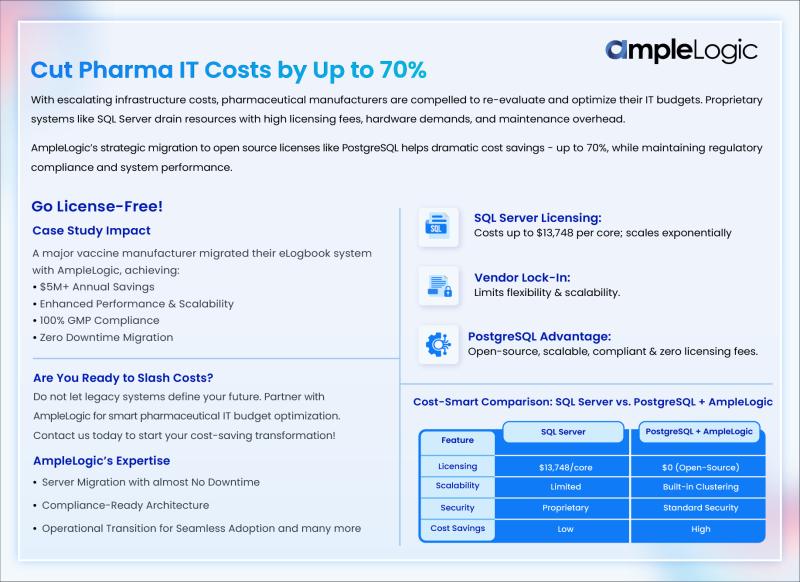
AmpleLogic helps Pharma Companies reduce IT spend by 70%
Pharmaceutical manufacturers are finding significant cost savings by migrating from expensive SQL Server licenses to PostgreSQL without compromising compliance or performance.
According to AmpleLogic's whitepaper, SQL Server Enterprise Edition costs approximately $13,748 per core, while Standard Edition costs around $3,586 per core. For medium to large pharmaceutical operations requiring multiple servers, these costs multiply rapidly. Beyond licensing fees, companies face additional costs for software assurance, specialized IT personnel, infrastructure upgrades, and…
More Releases for APQR
AmpleLogic's AI Delivers 60% Manual Effort Reduction in Pharma Operations
Pharma companies using AmpleLogic's low-code platform are reporting significant improvements in day-to-day efficiency, with AI reducing manual effort by up to 60% across training, investigations, documentation, and APQR review work. The gains reflect a core challenge inside regulated operations: teams spend more time understanding information than executing workflows.
"Pharma teams aren't short of data; they're short of time," says Manne V. Chowdary, Founder & CEO of AmpleLogic. "Our focus has been…
