Press release
Ensuring Analytical Precision: Lenacapavir Impurity Standards & Sodium Reference from Aquigen Bio
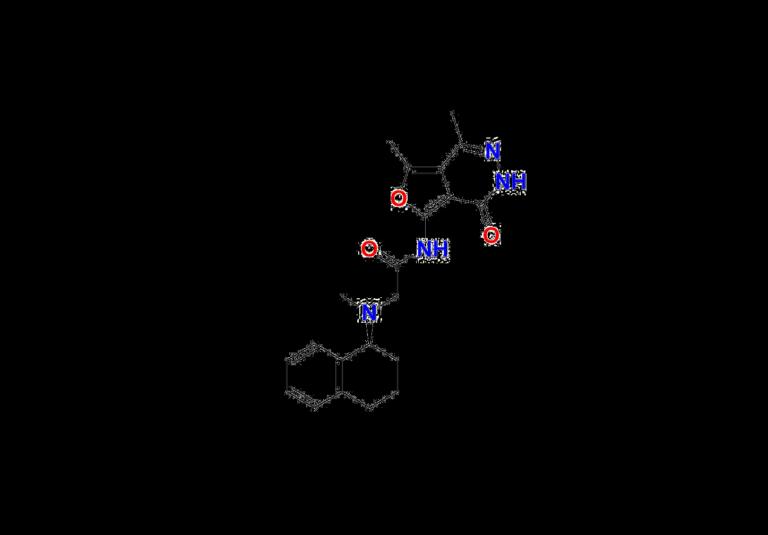
Ensuring Analytical Precision: Lenacapavir Impurity Standards & Sodium Reference from Aquigen BioAs lenacapavir (GS‐6207/Sunlenca) gains momentum in HIV prevention and treatment, providing accurate reference standards becomes critical. Aquigen Bio offers high-purity impurity standards-Lenacapavir Impurity‐7, Impurity‐9-plus Lenacapavir Sodium, all designed to support method development, quality control, and regulatory compliance in pharmaceutical workflows.
What is Lenacapavir?
Lenacapavir is a first-in-class HIV‐1 capsid inhibitor, approved by the FDA in December 2022, and marketed under names like Sunlenca and Yeztugo. Its mechanism targets multiple stages of viral replication, and it's administered in long-acting injectable or oral forms-providing biannual protection as a pre‐exposure prophylaxis (PrEP) option
Lenacapavir Impurity Standards: Impurity‐7 & Impurity‐9
Aquigen Bio provides well-characterized impurity standards from the lenacapavir synthesis pathway:
• Lenacapavir Impurity‐7
https://aquigenbio.com/product/lenacapavir-impurity-7/
A key process-related impurity. Essential for:
Method validation via HPLC, LC‐MS
Monitoring trace impurities in batches
Ensuring compliance with pharmacopeial and regulatory thresholds (e.g. ICH Q3A)
• Lenacapavir Impurity‐9
https://aquigenbio.com/product/lenacapavir-impurity-9/
A critical degradation impurity that forms during stability studies. Used for:
Accelerated and long-term stability analysis
Forced-degradation studies
Quantitative reporting during regulatory submissions
These impurity standards enable accurate detection and quantification-ensuring analysts meet ICH Q3A/B and US FDA regulatory criteria
Lenacapavir Sodium: High-Purity API Reference
https://aquigenbio.com/product/lenacapavir-sodium/
Lenacapavir Sodium (CAS 2283356‐12‐5), the sodium salt form, is available from Aquigen Bio with >98% purity and comprehensive documentation. Applications include:
Reference standard in QC laboratories
Calibration standard for method validation (AMV)
ANDA filing material and pharmacopeial traceable reference
Supplied with full coA, MS/NMR data, and stability profiles, this standard supports both routine QC checks and high-level regulatory procedures
Why High-Quality Lenacapavir Standards Are Essential
Analytical Integrity: Minimize false positives and misidentification
Regulatory Security: Ready for support in DMF/ANDA/NDA dossiers
Risk Reduction: Track degradation and synthesis impurities during shelf life
Workflow Efficiency: Pre-characterized standards save lab time and promote compliance
High-quality CRM standards reduce development delays and strengthen lab credentials in the eyes of reviewers.
Conclusion
With lenacapavir gaining prominence as a breakthrough HIV therapeutic, having reliable impurity and API standards is no longer optional-it's essential. Aquigen Bio delivers robust, validated materials that help pharmaceutical labs uphold regulatory standards, reduce risk, and accelerate development. Explore their catalog to secure your analytical advantage.
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Visit: www.aquigenbio.com
Aquigen Bio Sciences stands as India's premier resource for high-quality N-Nitroso impurity standards, impelling advancements in pharmaceutical research and manufacturing. Specializing in providing precise impurity standards - including degradation impurities, process impurities, and deuterated isotopes - Aquigen Bio Sciences empowers the industry to comply with the most rigorous global regulations. The contract research organization's commitment to precision and reliability makes it the trusted partner for addressing complex issues like N-Nitroso impurities in pharmaceuticals.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Ensuring Analytical Precision: Lenacapavir Impurity Standards & Sodium Reference from Aquigen Bio here
News-ID: 4085002 • Views: …
More Releases from Aquigen Bio Sciences
Elevate Pharmaceutical R&D with Aquigen BioSciences' Precision‐Grade Flibanser …
Flibanserin Impurity B is a reference standard used in pharmaceutical research and development. It is primarily applied during the analysis and validation of drug substances to identify, quantify, and control impurities that may be present in the final product. This impurity is associated with the parent compound, Flibanserin, a medication approved for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women.
Aquigen BioSciences offers Flibanserin Impurity B as a…

Estradiol Valerate EP Impurity A - Premium Reference Standard for Analytical Dev …
Estradiol Valerate EP Impurity A is a high-quality reference standard designed to meet the stringent requirements of pharmaceutical research, method validation, and quality control processes.
Explore Estradiol Valerate EP Impurity A :
https://aquigenbio.com/product/estradiol-valerate-ep-impurity-a/
Manufactured and characterized with precision, this impurity standard supports laboratories and manufacturers in achieving consistent, reliable, and reproducible results in critical analytical workflows.
With its exceptional purity and accurate characterization, Estradiol Valerate EP Impurity A plays a vital role…

High-Purity N-Nitroso Betahistine D3 for Precise Pharmaceutical Analysis | Deute …
Product Overview
N-Nitroso Betahistine D3 is a premium deuterated nitrosamine impurity standard, specifically developed for precise analytical testing in pharmaceutical laboratories. This reference standard is widely used for analytical method development, validation, and quality control processes to meet stringent regulatory guidelines. With exceptional purity, complete documentation, and reliable traceability, it is ideal for research, development, and compliance applications.
https://aquigenbio.com/product/n-nitroso-betahistine-d3/
Key Features and Benefits
Deuterated Design for Precision: The incorporation of deuterium improves mass spectrometric…

Aquigen Bio Strengthens Pharmaceutical Research with High-Purity Icatibant Impur …
Aquigen Bio, a trusted supplier of pharmaceutical reference standards, today announced the expansion of its Icatibant Impurity Standards portfolio, designed to support drug developers, analytical laboratories, and research organizations with reliable materials for impurity profiling and quality control.
Icatibant, a selective bradykinin B2 receptor antagonist, is widely used in the treatment of hereditary angioedema (HAE). Given its peptide-based structure, Icatibant is prone to the formation of impurities during synthesis and storage.…
More Releases for Lenacapavir
Global Human Immunodeficiency Virus Type-1 (HIV-1) Treatment Market to reach US$ …
Global Human Immunodeficiency Virus Type-1 (HIV-1) Treatment Market reached US$ 39,670.14 million in 2023 and is expected to reach US$ 62,752.56 million by 2031, growing at a CAGR of 5.9% during the forecast period 2025-2031.
HIV-1 is a retrovirus, belonging to a group of heterogeneous, lipid-enveloped RNA viruses. HIV-1 is the most common type of HIV (Human Immunodeficiency Virus) accounting for nearly 95% of the cases. The virus attacks the…
Lenacapavir Injection Market: Is Booming So Rapidly with Strategic Insights from …
Coherent Market Insights has released a report titled "Lenacapavir Injection Market: Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2025-2032", which includes market percentage records and a thorough enterprise analysis. As part of our Black Friday Limited-Time Discount, this premium research report is now available at up to 60% off, offering an exceptional opportunity for businesses, analysts, and stakeholders to access high-value insights at a significantly reduced cost. This report…
HIV-AIDS Testing: Latest Guidelines, Innovations, and Impact 2025
Overview
The HIV-AIDS Testing Market is the cornerstone of global health strategies to prevent transmission, initiate early treatment, and ultimately control the HIV epidemic. In 2025, major health agencies, including WHO and CDC, have introduced significant updates to HIV testing guidelines, simplifying access and amplifying the impact of diagnostic services worldwide.
Download Sample Report - https://www.theinsightpartners.com/sample/TIPRE00016713?utm_source=OpenPR&utm_medium=10844
Key Bullet Points
Updated Testing Guidelines: WHO and CDC now recommend various rapid and laboratory-based HIV tests, including…
HIV Drugs Market Poised to Hit $41.55 Billion by 2029 with Accelerating Growth T …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
HIV Drugs Market Size Growth Forecast: What to Expect by 2025?
The market size of HIV drugs has been experiencing a consistent surge in recent years. It is projected to increase from $35.33 billion in 2024 to $36.55 billion in 2025, with a compound annual growth rate (CAGR) of…
HIV Drugs Market to Reach USD 47.5 Billion by 2034 Amid Steady 4.7% CAGR Growth
The global HIV drugs market was valued at USD 28.6 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 4.7% from 2024 to 2034. Driven by rising awareness, increasing access to antiretroviral therapy, and ongoing advancements in treatment options, the market is expected to reach USD 47.5 billion by the end of 2034.
Rise in the number of patients suffering from HIV infection is…
Elevated Awareness Initiatives Driving HIV Drug Market Growth : An Emerging Driv …
The HIV Drugs Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Projected Growth of the HIV Drugs Market?
In recent times, there has been a consistent expansion in the market size of HIV drugs. The market, projected to rise from $34.91 billion in…
