Press release
Non-small Cell Lung Cancer Clinical Trials, Companies, Therapeutic Assessment, Therapies, Treatment Algorithm, and Pipeline Analysis | GlaxoSmithKline, Novartis, AstraZeneca, Eli Lilly and Company
DelveInsight's, "Non-Small-Cell Lung cancer (NSCLC) - Pipeline Insight, 2025," report provides comprehensive insights about 10+ companies and 10+ pipeline drugs in Non-Small-Cell Lung cancer (NSCLC) pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.DelveInsight reports that the Non-Small Cell Lung Cancer (NSCLC) treatment pipeline includes over 10 prominent companies actively developing more than 10 therapeutic candidates for the disease.
Non-small Cell Lung Cancer Overview:
Non-small-cell lung cancer (NSCLC) encompasses all types of epithelial lung cancers excluding small-cell lung cancer (SCLC) and accounts for roughly 85% of all lung cancer cases. NSCLC is mainly classified into three types:
a) Adenocarcinoma - This type originates in the mucus-producing cells within the lung's air sacs, usually in the outer regions of the lungs. It is the most common form of lung cancer in both smokers and nonsmokers, particularly in individuals under 45, and generally tends to grow more slowly than other lung cancers.
b) Squamous cell carcinoma - Arising in the cells lining the airways, this type makes up around 25% of lung cancers.
c) Large cell (undifferentiated) carcinoma - This form grows and spreads more aggressively, making it more difficult to treat, and accounts for about 10% of cases.
Treatment for non-small-cell adenocarcinoma depends largely on the stage of the disease, with surgery-often involving partial or complete removal of the lung-being a primary option if the cancer has not metastasized.
Request for a detailed insights report on Non-small Cell Lung Cancer pipeline insights @ https://www.delveinsight.com/report-store/non-small-cell-lung-cancer-nsclc-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
"Non-small Cell Lung Cancer Pipeline Insight 2025" report by DelveInsight provides a comprehensive analysis of the ongoing clinical development activities and growth prospects across the Non-small Cell Lung Cancer Therapeutics Market.
Key Takeaways from the Non-small Cell Lung Cancer Pipeline Report
*
DelveInsight's Non-small Cell Lung Cancer pipeline report depicts a robust space with 10+ active players working to develop 10+ pipeline therapies for Non-small Cell Lung Cancer treatment.
*
In September 2025, AbbVie announced that it had submitted a Biologics License Application (BLA) to the US Food and Drug Administration (FDA) for the accelerated approval of elotuzumab vedotin (Teliso-V) in adult patients with previously treated, locally advanced or metastatic epidermal growth factor receptor (EGFR) wild type, nonsquamous non-small cell lung cancer (NSCLC) exhibiting c-Met protein overexpression.
*
In August 2025, the FDA granted Fast Track designation to Deltacel (KB-GDT-01) in combination with low-dose radiation therapy for the potential treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose disease has progressed after two or more prior lines of standard-of-care treatments, including immune checkpoint inhibitors, platinum-based chemotherapy, and targeted therapy.
*
In July 2025, Nuvalent announced that the first patient had been dosed in the HEROEX-1 Phase Ia/Ib clinical trial of NVL-330, its novel HER2-selective inhibitor.
*
Key Non-small Cell Lung Cancer companies such as GlaxoSmithKline, Novartis, AstraZeneca, Eli Lilly and Company, Pfizer Inc., F. Hoffmann-La Roche Ltd., Merck, Millennium Pharmaceuticals, Inc., Boehringer Ingelheim Pharmaceuticals, Inc., Bristol Myers Squibb, Sanofi, IO Biotech, Transgene, Immutep S.A., Daiichi Sankyo, Inc., Regeneron Pharmaceuticals, Hanmi Pharmaceutical Company Limited, TYK Medicines, Inc, and others are evaluating new drugs for Non-small Cell Lung Cancer to improve the treatment landscape.
*
Promising Non-small Cell Lung Cancer pipeline therapies in various stages of development include AMG 510, CMP 001, and others.
Non-small Cell Lung Cancer Pipeline Analysis
The report provides insights into:
*
The report provides detailed insights into the key companies that are developing therapies in the Non-small Cell Lung Cancer Market.
*
The report also evaluates different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Non-small Cell Lung Cancer treatment.
*
It analyzes the key companies involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
It navigates the emerging drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement, and financing details for future advancement of the Non-small Cell Lung Cancer market.
Download our free sample page report on Non-small Cell Lung Cancer pipeline insights @ https://www.delveinsight.com/sample-request/non-small-cell-lung-cancer-nsclc-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
Non-small Cell Lung Cancer Emerging Drugs
*
AMG 510: Amgen
*
CMP 001: Cytos Biotechnology
Non-small Cell Lung Cancer Companies
More than 10 leading companies are actively developing treatments for Non-Small-Cell Lung Cancer (NSCLC). Among them, Amgen has drug candidates currently in the mid to late stages of development, specifically in Phase I clinical trials.
DelveInsight's report covers around 10+ products under different phases of clinical development like
*
Late stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I) along with the details of
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
Non-small Cell Lung Cancer pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
*
Intravenous
*
Subcutaneous
*
Oral
*
Intramuscular
Non-small Cell Lung Cancer Products have been categorized under various Molecule types such as
*
Monoclonal antibody
*
Small molecule
*
Peptide
Download Sample Pages to Get an in-depth Assessment of the Emerging Non-small Cell Lung Cancer Therapies and Key Companies: Non-small Cell Lung Cancer Clinical Trials and advancements @ https://www.delveinsight.com/sample-request/non-small-cell-lung-cancer-nsclc-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
Non-small Cell Lung Cancer Pipeline Therapeutic Assessment
- Non-small Cell Lung Cancer Assessment by Product Type
- Non-small Cell Lung Cancer By Stage
- Non-small Cell Lung Cancer Assessment by Route of Administration
- Non-small Cell Lung Cancer Assessment by Molecule Type
Download Non-small Cell Lung Cancer Sample report to know in detail about the Non-small Cell Lung Cancer treatment market @ Non-small Cell Lung Cancer Therapeutic Assessment @ https://www.delveinsight.com/report-store/non-small-cell-lung-cancer-nsclc-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
Table of Content
1. Report Introduction
2. Executive Summary
3. Non-small Cell Lung Cancer Current Treatment Patterns
4. Non-small Cell Lung Cancer - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Non-small Cell Lung Cancer Late-Stage Products (Phase-III)
7. Non-small Cell Lung Cancer Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Non-small Cell Lung Cancer Discontinued Products
13. Non-small Cell Lung Cancer Product Profiles
14. Non-small Cell Lung Cancer Key Companies
15. Non-small Cell Lung Cancer Key Products
16. Dormant and Discontinued Products
17. Non-small Cell Lung Cancer Unmet Needs
18. Non-small Cell Lung Cancer Future Perspectives
19. Non-small Cell Lung Cancer Analyst Review
20. Appendix
21. Report Methodology
Request the Sample PDF to Get Detailed Insights About the Non-small Cell Lung Cancer Pipeline Reports Offerings: https://www.delveinsight.com/sample-request/non-small-cell-lung-cancer-nsclc-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=nonsmall-cell-lung-cancer-clinical-trials-companies-therapeutic-assessment-therapies-treatment-algorithm-and-pipeline-analysis-glaxosmithkline-novartis-astrazeneca-eli-lilly-and-company]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Non-small Cell Lung Cancer Clinical Trials, Companies, Therapeutic Assessment, Therapies, Treatment Algorithm, and Pipeline Analysis | GlaxoSmithKline, Novartis, AstraZeneca, Eli Lilly and Company here
News-ID: 4084080 • Views: …
More Releases from ABNewswire
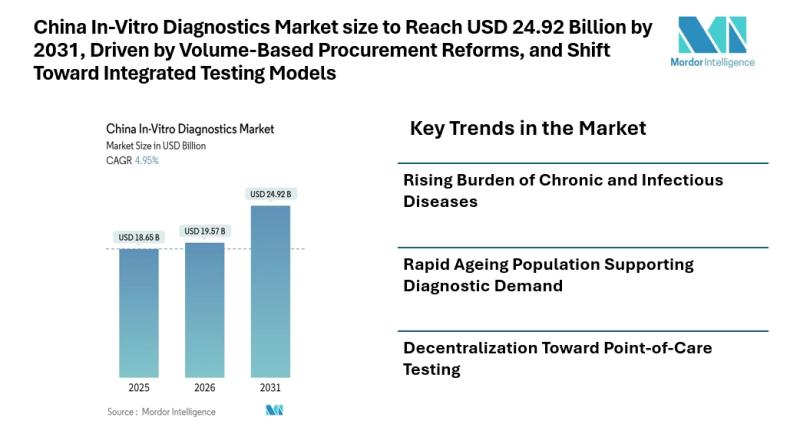
China In-Vitro Diagnostics Market size to Reach USD 24.92 Billion by 2031, Drive …
Mordor Intelligence has published a new report on the china in-vitro diagnostics market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Introduction
According to Mordor Intelligence, the china in-vitro diagnostics market size [https://www.mordorintelligence.com/industry-reports/china-in-vitro-diagnostics-market?utm_source=abnewswire] is projected to reach USD 24.92 billion by 2031, growing from USD 19.57 billion in 2026 at a CAGR of 4.95% during the forecast period. The china in-vitro diagnostics market size reflects steady expansion supported by…

Hyaluronic Acid Market Size to Reach USD 4.07 Billion by 2030 - Mordor Intellige …
Mordor Intelligence has released an in-depth analysis of the hyaluronic acid market, outlining expanding cosmetic, orthopedic, and pharmaceutical applications driving global demand.
Hyaluronic Acid Market Overview
According to Mordor Intelligence, the global hyaluronic acid market size [https://www.mordorintelligence.com/industry-reports/hyaluronic-acid-market?utm_source=abnewswire] reached USD 2.84 billion in 2025 and is projected to grow to USD 4.07 billion by 2030, registering a CAGR of 7.46% during the forecast period.
The strong hyaluronic acid market growth is supported by:
* Increasing…

Scott Bryant Unveils Moon Valley's "Best Value" Listing in Hillcrest East; Signa …
Bryant Real Estate Leverages Data-Driven Performance Metrics to Position New Hillcrest East Property as the Region's Premier Investment Opportunity
PHOENIX, AZ - Scott Bryant, Founder and Team Leader of Bryant Real Estate and a top-performing agent with Keller Williams, has announced the debut of a landmark listing in the Hillcrest East subdivision of Moon Valley. Positioned as "Moon Valley's Best Deal," the property is being introduced at a strategic price point…

Jennifer Rollin Named Best Individual Therapist in Best of Bethesda Awards
Bethesda, MD, USA - Jennifer Rollin, LCSW-C, eating disorder therapist and founder of The Eating Disorder Center, has been named Best Individual Therapist in the 2025 Best of Bethesda Awards. She was selected from among therapists across Montgomery County, Maryland and Upper Northwest Washington, D.C., an honor that reflects both community support and her longstanding commitment to helping individuals recover from eating disorders.
Jennifer Rollin provides eating disorder therapy [https://www.theeatingdisordercenter.com/eatingdisordertherapyrockvilleservices.html] in…
More Releases for Lung
Lung Cancer Prevalence Driving The Lung Cancer Drugs Market: Core Growth Enabler …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
What Will the Lung Cancer Drugs Industry Market Size Be by 2025?
The market for lung cancer medications has seen a swift expansion in previous years. The market size is projected to escalate from $47.94 billion in 2024 to $54.13 billion in 2025, with a compound annual growth rate…
Lung Cancer Prevalence Driving The Lung Cancer Drugs Market: An Emerging Driver …
The Lending And Payments Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Projected Growth of the Lending And Payments Market?
The lending and payments market has experienced strong growth in recent years. It is set to rise from $12,326.44 billion in 2024 to…
Transforming the Lung Cancer Drugs Market in 2025: Lung Cancer Prevalence Drivin …
What Is the Expected Size and Growth Rate of the Lung Cancer Drugs Market?
The market size for lung cancer medications has seen swift expansion in the recent past. Its growth is projected to surge from $47.94 billion in 2024 to $54.13 billion in 2025, marking a compound annual growth rate (CAGR) of 12.9%. Factors contributing to the historical period's growth include epidemiology and demographics, regulatory authorizations, healthcare system infrastructure, and…
Transforming the Lung Cancer Drugs Market in 2025: Lung Cancer Prevalence Drivin …
What Is the Expected Size and Growth Rate of the Lung Cancer Drugs Market?
The market size for lung cancer medications has seen swift expansion in the recent past. Its growth is projected to surge from $47.94 billion in 2024 to $54.13 billion in 2025, marking a compound annual growth rate (CAGR) of 12.9%. Factors contributing to the historical period's growth include epidemiology and demographics, regulatory authorizations, healthcare system infrastructure, and…
Lung Cancer Screening Market - Empowering Proactive Lung Health: The Future of C …
Newark, New Castle, USA: The "Lung Cancer Screening Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Lung Cancer Screening Market: https://www.growthplusreports.com/report/lung-cancer-screening-market/7787
This latest report researches the industry structure,…
Artificial Lung Market May Set New Growth Story | Breethe, Lung Biotechnology, M …
A new research document is added in HTF MI database of 37 pages, titled as 'Artificial Lung - Medical Devices Pipeline Assessment, 2020' with detailed analysis, Competitive landscape, forecast and strategies. The study covers important players/vendors such as ALung Technologies Inc, Breethe, Inc., Case Western Reserve University, Lung Biotechnology PBC, MC3 Inc, McGowan Institute for Regenerative , Medicine, Miromatrix Medical Inc, The Charles Stark Draper Laboratory Inc, U.S. Ann Arbor…
