Press release
Clinical Trial Management Systems Market Size, Share & Analysis - Key Drivers and Regional Insights
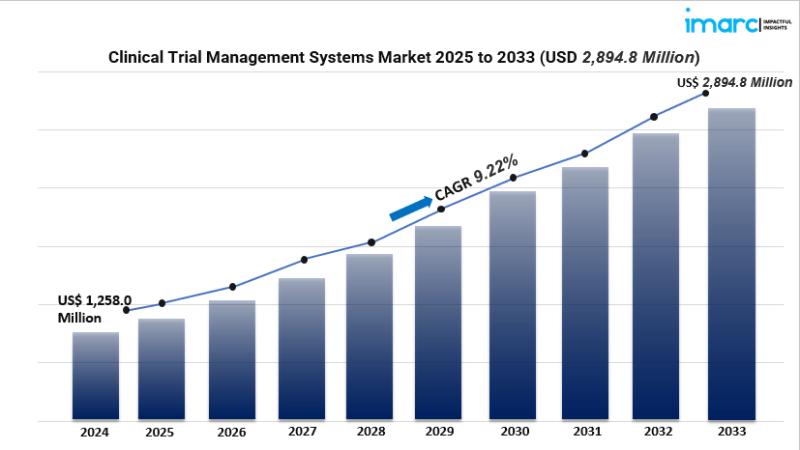
MARKET OVERVIEWThe clinical trial management systems market is rapidly expanding, driven by the increasing complexity of multisite trials, robust regulatory demands, and the rising adoption of cloud-based platforms for remote collaboration and patient-centric designs. In 2024, the global market reached USD 1,258 million, and it is projected to grow to USD 2,894.8 million by 2033, at a healthy 9.22 % CAGR. These trends highlight efficiency gains and improved data quality as key market benefits.
STUDY ASSUMPTION YEARS
BASE YEAR: 2024
HISTORICAL YEAR: 2019-2024
FORECAST YEAR: 2025-2033
CLINICAL TRIAL MANAGEMENT SYSTEMS MARKET KEY TAKEAWAYS
• Market valued at USD 1,258 million in 2024, forecast to nearly double to USD 2,894.8 million by 2033 at a 9.22 % CAGR
• Software components lead over services in revenue share
• Web based CTMS dominates the deployment segment
• Pharmaceutical & biotechnology firms are the primary end users
• North America leads regionally; Asia Pacific and Latin America are fast-emerging markets
• Growth fueled by rising trial complexity, decentralized trials, and integration trends
MARKET GROWTH FACTORS
1. Technology & Cloud Adoption
The rapid evolution of software technologies-particularly cloud-based solutions, artificial intelligence (AI), machine learning (ML), and real-world data integration-plays a pivotal role in the growth of the Clinical Trial Management System (CTMS) market. Transitioning to cloud-native platforms allows for smooth remote collaboration among trial sites worldwide, which boosts trial efficiency and cuts costs. AI and ML are game-changers for protocol design, patient recruitment, and risk-based monitoring, tackling the increasing complexity of trials and the demand for higher data quality. Additionally, integrating with Electronic Data Capture (EDC), electronic Patient-Reported Outcomes (ePRO), Randomization and Trial Supply Management (RTSM), and other eClinical tools creates a cohesive ecosystem, giving stakeholders access to real-time insights. As decentralized and virtual trial models become more popular, the need for flexible, interoperable CTMS platforms that can scale and integrate is on the rise.
2. Regulatory & Compliance Requirements
On the regulatory front, heightened oversight and strict compliance requirements worldwide are significant factors driving CTMS adoption. Regulatory bodies expect transparent, auditable, and standardized data capture workflows-areas where CTMS truly shines. Effective document management, version control, protocol adherence tracking, and adverse event reporting are essential, especially for large multinational trials. CTMS platforms simplify these tasks by incorporating compliance checks and standardized workflows, which help minimize audit risks and reduce administrative burdens. This is especially crucial in North America and Europe, where regulatory standards demand top-notch data systems. The increasing focus on patient safety and data integrity further solidifies CTMS as a vital technological investment in clinical research operations.
3. Rising Demand & Trial Complexity
The rise of complex clinical trials-like multisite global studies, precision medicine, and decentralized models-is driving a greater use of Clinical Trial Management Systems (CTMS). With trial sites often scattered, there's a real need for coordinated planning, scheduling, budget tracking, and resource allocation, all of which CTMS platforms handle efficiently. As the demand grows in areas like oncology, rare diseases, and chronic conditions, we see a need for data-rich protocols, sophisticated patient enrollment processes, and real-time monitoring capabilities. The global trend towards patient-centric trial design highlights the importance of participant engagement platforms that are integrated within CTMS ecosystems. Additionally, emerging markets in the Asia Pacific and Latin America are ramping up their outsourcing to Contract Research Organizations (CROs) and investing in trial infrastructure, which is further boosting CTMS deployment. This all points to the vital role CTMS plays in streamlining trial operations and adapting to the increasing complexity of clinical research around the world.
Request for a sample copy of this report: https://www.imarcgroup.com/clinical-trial-management-systems-market/requestsample
MARKET SEGMENTATION
Breakup by Component
• Software - standalone management platforms
• Services - implementation, customization, consulting
Breakup by Deployment Mode
• Web based CTMS - browser based access, real time updates
• On premise - locally hosted installations
• Cloud based CTMS - SaaS deployments with scalable infrastructure
Breakup by End User
• Pharmaceutical and Biotechnology Firms - sponsors & innovators
• Contract Research Organizations - outsourced trial management
• Others - hospitals, academic medical centers, research institutions
Breakup by Region
• North America (United States, Canada)
• Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
• Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
• Latin America (Brazil, Mexico, Others)
• Middle East and Africa
REGIONAL INSIGHTS
In North America, the CTMS market is thriving, thanks to a robust healthcare infrastructure, significant investment in clinical research, and strong regulatory frameworks. This region is at the forefront of adopting cloud-based solutions and decentralized trials. With its well-established pharmaceutical and biotech industries pushing for modernization, North America stands out as the fastest-growing and largest market globally.
RECENT DEVELOPMENTS & NEWS
The web-based CTMS segment is leading the charge, reflecting a clear shift towards more accessible, browser-based platforms. Key innovations include better integration with Electronic Data Capture (EDC) and virtual trial platforms, along with advanced risk-based monitoring features. There's also a noticeable increase in the adoption of decentralized and virtual trial modules within CTMS platforms, which enhances patient recruitment and trial flexibility. The focus on interconnectivity-especially through APIs and cloud services-improves seamless data flow across systems, highlighting the market's commitment to creating unified eClinical ecosystems.
KEY PLAYERS
• Advarra Inc.
• ArisGlobal LLC
• BioClinica Inc.
• DataTrak International Inc.
• DZS Clinical Services Inc. (WDB Holdings Co. Ltd.)
• International Business Machines Corporation
• Medidata Solutions Inc. (Dassault Systèmes SE)
• MedNet Solutions Inc.
• Oracle Corporation
• Parexel International Corporation
• RealTime Software Solutions LLC
• Veeva Systems Inc.
Ask Analyst for Customization: https://www.imarcgroup.com/request?type=report&id=4984&flag=C
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No: +1-631-791-1145
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provides a comprehensive suite of market entry and expansion services. IMARC offerings include a thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape, and benchmarking analyses, pricing and cost research, and procurement research.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Management Systems Market Size, Share & Analysis - Key Drivers and Regional Insights here
News-ID: 4065189 • Views: …
More Releases from IMARC Group

Global Liquid CO2 Prices Q4 2025: Industry Demand, Regional Breakdown & Forecast …
USA Liquid Carbon Dioxide Prices Movement Q4 2025:
During Q4 2025, liquid carbon dioxide prices in the USA reached USD 837/MT, reflecting tight supply conditions and strong demand from food processing, beverage carbonation, and industrial applications. Seasonal consumption, maintenance shutdowns at ammonia plants, and elevated logistics costs contributed to firm pricing across the domestic market.
Get the Real-Time Prices Analysis: https://www.imarcgroup.com/liquid-carbon-dioxide-pricing-report/requestsample
Note: The analysis can be tailored to align with the customer's specific…

Titanium Dioxide Production Cost Analysis Report 2026: Machinery and Technology …
Setting up a titanium dioxide manufacturing plant involves strategic planning, substantial capital investment, and comprehensive understanding of production technologies. This essential inorganic compound serves paint and coatings, plastics, and specialty chemical industries. Success requires careful site selection, efficient chloride or sulfate process operations, advanced reactor systems, reliable raw material sourcing, and compliance with environmental and safety regulations to ensure profitable and sustainable operations.
IMARC Group's report, "Titanium Dioxide Production Plant Project…

TFT LCD Manufacturing Plant DPR & Unit Setup 2026: Demand Analysis and Project C …
Setting up a TFT LCD manufacturing plant involves strategic planning, substantial capital investment, and comprehensive understanding of display panel production technologies. TFT LCD panels are essential components in consumer electronics, automotive displays, industrial monitors, and medical equipment. Success requires careful site selection, advanced thin-film transistor deposition processes, precision photolithography systems, reliable raw material sourcing, and compliance with environmental and safety regulations to ensure profitable and sustainable operations.
IMARC Group's report, "TFT…
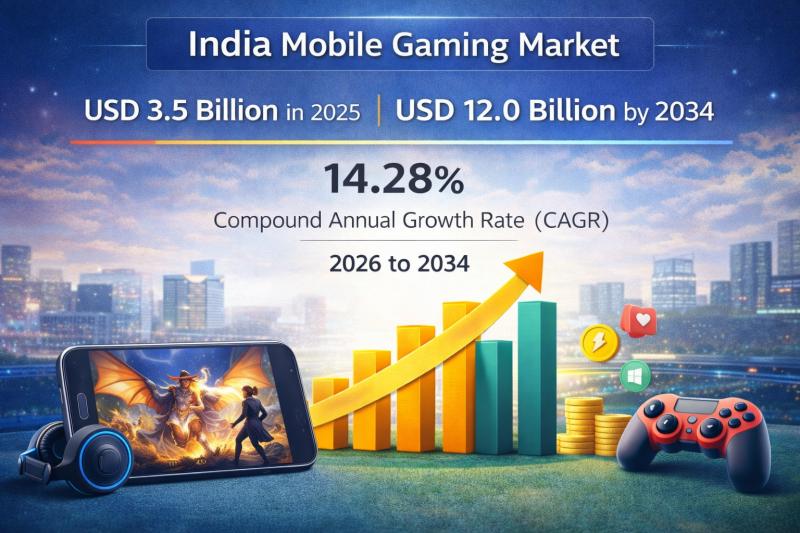
India Mobile Gaming Market 2026-2034: Explosive Growth, Revenue Forecast & Inves …
India Mobile Gaming Market Overview: 2026-2034
According to IMARC Group's report titled "India Mobile Gaming Market Size, Share, Trends and Forecast by Monetization Type, Platform, Game Type, and Region, 2026-2034" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
How Big is the India Mobile Gaming Industry?
The India mobile gaming market was valued at USD 3.5 Billion in 2025 and is expected to reach…
More Releases for CTMS
Key Trends Shaping the Future Clinical Trial Management System CTMS Market From …
What Is the Estimated Market Size and Growth Rate for the Clinical Trial Management System CTMS Market?
The clinical trial management system (CTMS) market has experienced rapid growth in recent years. It is expected to grow from $1.41 billion in 2024 to $1.61 billion in 2025, with a CAGR of 14.2%. Growth factors include increasing clinical trial complexity, a rise in global clinical trials, regulatory compliance demands, an increasing focus on…
Clinical Trial Management System (CTMS) Market: Growth, Trends, Opportunities, a …
Introduction
A Clinical Trial Management System (CTMS) is an integrated software platform designed to streamline and manage the planning, execution, and monitoring of clinical trials. It helps healthcare organizations, pharmaceutical companies, research institutions, and contract research organizations (CROs) efficiently oversee the complex and data-driven processes of clinical trials. The CTMS enables management of trial activities such as patient recruitment, data collection, regulatory compliance, budgeting, and reporting.
Clinical trials are a critical component…
Clinical Trial Management System (CTMS) Market: Growth, Opportunities, and Chall …
Introduction
The Clinical Trial Management System (CTMS) market is a crucial segment of the global healthcare and pharmaceutical industries, which supports the efficient and accurate management of clinical trials. CTMS refers to software systems used by pharmaceutical, biotechnology, and contract research organizations (CROs) to streamline the planning, tracking, and management of clinical trials. These systems provide centralized data management for clinical trial activities, which helps improve the speed, quality, and compliance…
Clinical Trial Management System (CTMS) Global Market Report 2024 - Clinical Tri …
"The Business Research Company recently released a comprehensive report on the Global Clinical Trial Management System (CTMS) Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
According to The Business Research Company's, The…
Clinical Trial Management System (CTMS) Market Scope & Future Opportunities Till …
An exclusive Clinical Trial Management System (CTMS) Market research report has been fabricated through the in depth analysis of the market dynamics across five regions including North America, Europe, South America, Asia-Pacific, Middle East and Africa. The segmentation of the market by components, end users, and region was done based on the thorough market analysis and validation through extensive primary inputs from industry experts (key opinion leaders of companies, and…
Latest Clinical Trial (CTMS) Market 2022 | Detailed Report
ReportsnReports publishes the report titled Clinical Trial (CTMS) that presents a 360-degree overview of the market under one roof. The report is developed with the meticulous efforts of an enthusiastic and experienced team of experts, analyts, and researchers that makes the report a valuable asset for stakeholders to make robust decisions. This report also provides an in-depth overview of product type, specification, technology, and production analysis considering vital factors like…