Press release
Vitamins and Herbal Dietary Supplements Market to be Driven by Growing Demand for Indigenous Medicine
The top performing products in the U.S. dietary supplement market are exhibiting double-digit growth rate thanks to heightened consumer focus on health, improved industry regulation, and industry trends that are focused on globalization.How safe are herbal dietary supplements?
The manufacture of herbal dietary supplements is regulated by the FDA in the United States, but is not subject to the same scientific scrutiny or regulations as food or drugs. For instance, herbal supplements manufacturers are required to follow good manufacturing practices in order to ensure that supplements are processed under uniform conditions and meet quality standards but they do not need approval from the Food and Drug Administration (FDA) before launching their products in the market.
Nevertheless, the FDA regulates both dietary ingredients and finished dietary supplements. The FDA oversees dietary supplements under a different set of mandates than those that cover conventional foods and drugs. These are regulated under the Dietary Supplement Health and Education Act of 1994 (DSHEA), which states that:
Dietary ingredients and dietary supplements that are adulterated or misbranded are prohibited to be marketed by product manufacturers and distributors.
FDA is responsible for taking action against any misbranded or adulterated dietary supplements after it is introduced in the market.
Get Free PDF Brochure for more Professional and Technical Insights: http://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=10913
What are the core factors driving the growth of the vitamins and herbal dietary supplements market?
The demand for safe and naturally derived curative options persists among consumers. Consumers are more inclined towards the purchase of herbal products combined with government support for promoting the use of traditional medicine. For instance, Traditional Chinese Medicine (TCM), Ayurveda, and several other indigenous medicines are increasing their impact. Furthermore, the rising healthcare cost is also favoring the growth of the vitamins and herbal dietary supplements market as consumers are seeking alternate options for medical conditions.
There are several reasons for taking vitamin supplements, such as over-the-counter multivitamins. As per the American Academy of Family Physicians (AAFP), vitamin supplements are recommended to be taken for:
Certain health conditions.
Individuals who eat a vegetarian or vegan diet.
Pregnant or breastfeeding women.
Are herbs and botanicals regulated by legislations in the European Union?
Yes. Traditional herbal medicines for human use are as much regulated as pharmaceutical products. A simplified registration procedure for the pharmaceutical legislation of traditional herbal medicinal products was introduced in 2004 that overcame the difficulties encountered by the member states earlier. The objective of the simplified registration procedure is to safeguard public health and eliminate misinterpretations and uncertainties about traditional botanical products that existed among the member states and allow the free movement of these products by introducing harmonized rules in the region. This has reverberated in the form of increased sales of herbal supplements and botanicals and many new companies have entered the dietary supplements space.
The vitamins, minerals, and health supplements market is highly fragmented and has immense opportunities for mergers and acquisitions. In recent years, both pharmaceutical companies and makers of consumer packaged goods (CPG) are gearing up to expand their consumer health division, which suggests that acquisitions in this space will continue to happen.
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants, use proprietary data sources and various tools and techniques to gather, and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Transparency Market Research
US Office Contact
90 State Street, Suite 700
Albany, NY 12207
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: http://www.transparencymarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Vitamins and Herbal Dietary Supplements Market to be Driven by Growing Demand for Indigenous Medicine here
News-ID: 403339 • Views: …
More Releases from Transparency Market Research

Medium Voltage Fuse Market Outlook 2031: Global Market to Reach US$ 1.8 Billion …
The global medium voltage fuse market is steadily transitioning from a traditional grid-protection niche to a strategic enabler of modern power systems. Rising investments in renewable energy integration, large-scale electrification programs, and infrastructure upgrades are reshaping demand patterns worldwide. Medium voltage fuses-typically rated between 1 kV and 35 kV-are no longer viewed as passive safety components; instead, they are increasingly recognized as critical assets for grid stability, asset protection, and…
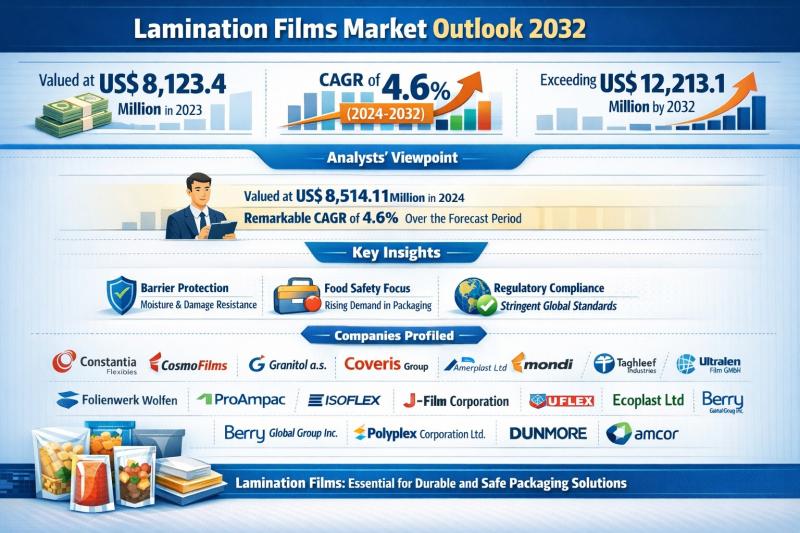
Lamination Films Market Outlook 2032: Global Industry Size to Surpass US$ 12.21 …
The global lamination films market was valued at US$ 8,123.4 million in 2023 and is forecast to reach US$ 8,514.1 million in 2024. Over the forecast period from 2024 to 2032, the market is projected to expand at a compound annual growth rate (CAGR) of 4.6%, ultimately exceeding US$ 12,213.1 million by 2032. This steady growth trajectory reflects the indispensable role of lamination films in modern packaging ecosystems across food,…

Global Curcumin Market Outlook 2031: Natural Antioxidant Demand, Regional Growth …
The global curcumin market is entering a structurally strong growth phase, underpinned by rising consumer preference for natural, plant-based ingredients and increasing clinical validation of curcumin's antioxidant and anti-inflammatory properties. As consumers shift away from synthetic additives and chemical-based therapeutics, curcumin is emerging as a high-value bioactive ingredient across , functional foods, cosmetics, and pharmaceuticals. Premiumization trends in organic products, combined with regulatory validation from food safety authorities, are expected…

Hemoglobin A1c Testing Devices Market to be Worth More Than USD 3.3 Bn by 2034 - …
The global Hemoglobin A1c (HbA1c) Testing Devices Market was valued at US$ 1.4 Bn in 2023 and is projected to expand at a CAGR of 7.7% from 2024 to 2034, reaching more than US$ 3.3 Bn by the end of 2034. The market growth is primarily attributed to the increasing global burden of diabetes, growing awareness about disease management, and technological advancements in diagnostic devices.
Get a concise overview of key…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
