Press release
Pharmacovigilance Market Size, Share, Growth, and Demand Forecast 2025-2033
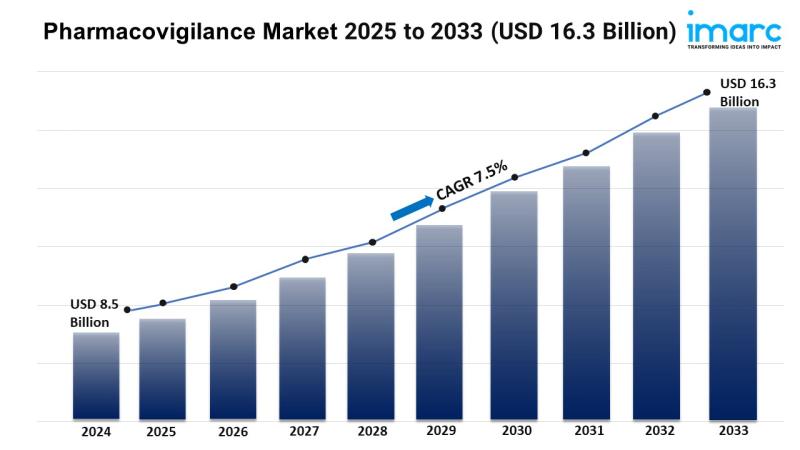
Market Overview:The pharmacovigilance market is experiencing rapid growth, driven by rising drug safety concerns, ai and automation adoption, and emerging markets expansion. According to IMARC Group's latest research publication, "Pharmacovigilance Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global pharmacovigilance market size was valued at USD 8.5 Billion in 2024. Looking forward, IMARC Group estimates the market to reach USD 16.3 Billion by 2033, exhibiting a CAGR of 7.5% from 2025-2033.
This detailed analysis primarily encompasses industry size, business trends, market share, key growth factors, and regional forecasts. The report offers a comprehensive overview and integrates research findings, market assessments, and data from different sources. It also includes pivotal market dynamics like drivers and challenges, while also highlighting growth opportunities, financial insights, technological improvements, emerging trends, and innovations. Besides this, the report provides regional market evaluation, along with a competitive landscape analysis.
Grab a sample PDF of this report: https://www.imarcgroup.com/pharmacovigilance-market/requestsample
Our report includes:
● Market Dynamics
● Market Trends And Market Outlook
● Competitive Analysis
● Industry Segmentation
● Strategic Recommendations
Factors Affecting the Growth of the Pharmacovigilance Industry:
● Rising Drug Safety Concerns:
The increasing complexity of drug therapies and growing awareness of adverse drug reactions (ADRs) are driving demand for robust pharmacovigilance (PV) systems. Regulatory agencies like the FDA and EMA are enforcing stricter safety monitoring requirements, compelling pharmaceutical companies to invest in advanced PV solutions. The rise in biologic and biosimilar approvals further amplifies the need for proactive risk management. As a result, the PV market is expanding, with a focus on real-world data (RWD) and AI-driven analytics to enhance drug safety surveillance.
● AI and Automation Adoption:
Pharmacovigilance is undergoing a digital transformation, with AI and automation revolutionizing case processing and signal detection. Machine learning algorithms can analyze vast datasets to identify potential safety signals faster than traditional methods, reducing manual workload and improving accuracy. Companies are increasingly adopting cloud-based PV platforms to streamline workflows and ensure compliance. This shift not only boosts efficiency but also cuts operational costs, making AI a key growth driver in the PV market.
● Emerging Markets Expansion:
Developing regions like Asia-Pacific and Latin America are witnessing rapid growth in pharmacovigilance demand due to increasing clinical trials and local drug production. Governments in these regions are strengthening regulatory frameworks to align with global standards, creating opportunities for PV service providers. The rise in chronic diseases and access to healthcare further fuels the need for drug safety monitoring. As a result, multinational pharma companies are expanding their PV operations in these markets, driving regional market growth.
Buy Full Report: https://www.imarcgroup.com/checkout?id=3609&method=1670
Leading Companies Operating in the Global Pharmacovigilance Industry:
● ArisGlobal LLC
● BioClinica Inc. (Cinven Partners LLP)
● Capgemini
● Cognizant
● International Business Machines Corporation
● ICON plc.
● IQVIA Inc.
● ITClinical
Parexel International Corporation and Wipro Limited.
Pharmacovigilance Market Report Segmentation:
By Service Provider:
● In-house
● Contract Outsourcing
Contract outsourcing represents the largest segment due to the reliance of pharmaceutical companies on specialized service providers to manage pharmacovigilance activities, allowing them to focus on core operations while leveraging external expertise and cost efficiencies.
By Product Life Cycle:
● Pre-clinical
● Phase I
● Phase II
● Phase III
● Phase IV
Phase IV accounts for the majority of the market share as post-market surveillance becomes increasingly critical for monitoring drug safety and effectiveness in real-world settings, ensuring compliance with regulatory requirements and addressing emerging safety concerns post-approval.
By Type:
● Spontaneous Reporting
● Intensified ADR Reporting
● Targeted Spontaneous Reporting
● Cohort Event Monitoring
● EHR Mining
Spontaneous reporting exhibits a clear dominance in the market owing to its rising utilization in collecting adverse event data, leveraging healthcare professionals, patients, and other stakeholders to report adverse reactions voluntarily.
By Process Flow:
● Case Data Management
● Case Logging
● Case Data Analysis
● Medical Reviewing and Reporting
● Signal Detection
● Adverse Event Logging
● Adverse Event Analysis
● Adverse Event Review and Reporting
● Risk Management System
● Risk Evaluation System
● Risk Mitigation System
Signal detection holds the biggest market share driven by its pivotal role in identifying potential safety concerns by analyzing pharmacovigilance data to detect statistical associations between drugs and adverse events, enabling proactive risk management and regulatory compliance.
By Therapeutic Area:
● Oncology
● Neurology
● Cardiology
● Respiratory Systems
● Others
Oncology dominates the market, with escalating demand for robust pharmacovigilance strategies to monitor the safety profiles of anticancer drugs and manage unique safety challenges associated with oncology treatments.
By End Use:
● Pharmaceuticals Companies
● Biotechnology Companies
● Medical Device Companies
● Others
Pharmaceutical companies represent the largest segment, which can be attributed to their crucial role in developing, manufacturing, and marketing drugs, necessitating comprehensive pharmacovigilance programs to ensure drug safety and regulatory compliance throughout the product lifecycle.
Regional Insights:
● North America (United States, Canada)
● Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
● Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
● Latin America (Brazil, Mexico, Others)
● Middle East and Africa
North America enjoys the leading position in the pharmacovigilance market on account of its well-established regulatory framework, advanced healthcare infrastructure, high pharmaceutical expenditure, and presence of major pharmaceutical companies.
Ask Analyst for Sample Report: https://www.imarcgroup.com/request?type=report&id=3609&flag=C
Research Methodology:
The report employs a comprehensive research methodology, combining primary and secondary data sources to validate findings. It includes market assessments, surveys, expert opinions, and data triangulation techniques to ensure accuracy and reliability.
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmacovigilance Market Size, Share, Growth, and Demand Forecast 2025-2033 here
News-ID: 3985075 • Views: …
More Releases from IMARC Group
India Women Apparel Market Outlook 2026-2034: Fashion Trends, Industry Share & O …
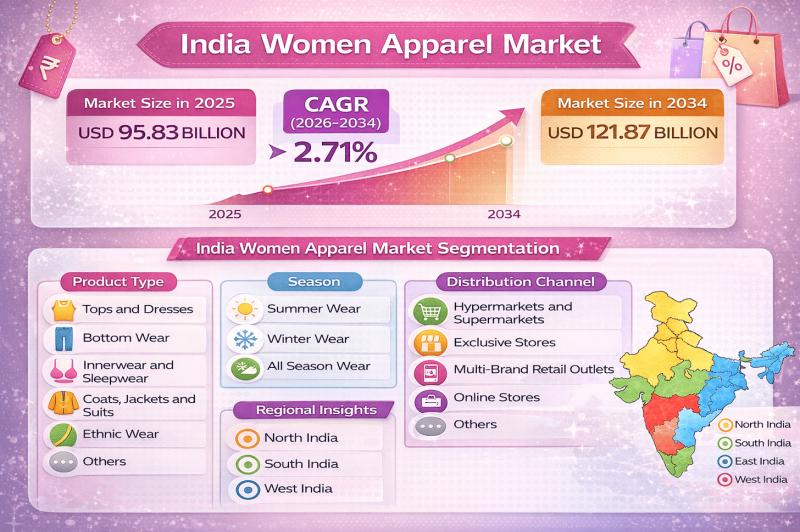
According to IMARC Group's report titled "India Women Apparel Market Size, Share, Trends and Forecast by Product Type, Season, Distribution Channel, and Region, 2026-2034" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
India Women Apparel Market Outlook
The India women apparel market size was valued at USD 95.83 Billion in 2025 and is projected to reach USD 121.87 Billion by 2034, growing at…

India Women Apparel Market Outlook 2026-2034: Fashion Trends, Industry Share & O …
According to IMARC Group's report titled "India Women Apparel Market Size, Share, Trends and Forecast by Product Type, Season, Distribution Channel, and Region, 2026-2034" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
India Women Apparel Market Outlook
The India women apparel market size was valued at USD 95.83 Billion in 2025 and is projected to reach USD 121.87 Billion by 2034, growing at…
Australia Reverse Logistics Market Projected to Reach USD 21,448.0 Million by 20 …
Market Overview
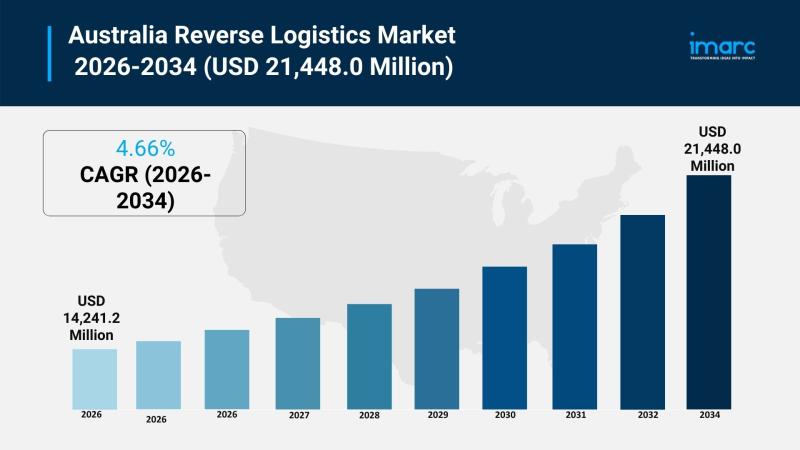
The Australia reverse logistics market size reached USD 14,241.2 Million in 2025 and is projected to reach USD 21,448.0 Million by 2034, growing at a CAGR of 4.66% during 2026-2034. This expansion is driven by the rise in e-commerce platforms, environmental sustainability efforts, and the integration of advanced technologies in logistics operations. The market encompasses return types, services, end users, and regional segments across Australia. For more details, visit…

Global Hummus Market Report 2026-2034: Growth, Trends, Packaging, Channels & Reg …
The global hummus market size reached USD 4.7 Billion in 2025 and is anticipated to reach USD 9.1 Billion by 2034, reflecting a CAGR of 7.50% during the forecast period 2026-2034. This growth is driven by increasing lifestyle diseases, rising health-conscious consumers, and escalating demand for plant-based proteins. The popularity of hummus as a substitute for traditional condiments further supports market expansion.
Study Assumption Years
Base Year: 2025
Historical Period: 2020-2025
Forecast Period:…
More Releases for Pharmacovigilance
Top Pharmacovigilance Companies Analysis By 2031
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
Download PDF Copy @ https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpenPR&utm_medium=10379
The List of Companies
• #Accentures
• Bristol-Myers Squibb Company
• Linical Accelovance
• Cognizant
• Covance Inc.
• F. Hoffmann-La Roche Ltd.
• GlaxoSmithKline plc.
• ICON plc
• Capgemini (IGATE Corporation)
Clinical…
Pharmacovigilance - Scope and Research Methodology
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The Pharmacovigilance Market report covers analysis by Clinical Trial Phase (Pre-Clinical, Phase I, Phase II, Phase III, and Phase IV), Service Provider (In-House and Contract Outsourcing), Type of Method (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event…
Pharmacovigilance World 2025 Conference & Expo
We are delighted to welcome you to the Pharmacovigilance World 2025, and we are confident that your active participation will contribute to the advancement of drug safety practices. Together, let us strive towards a safer and more vigilant healthcare system that prioritizes patient well-being and ensures the continued benefit of medications worldwide.
As medical science advances, so does our understanding of drug safety and the need for vigilance when it comes…
Top Factor Driving Pharmacovigilance Market Growth in 2025: Research And Develop …
How Are the key drivers contributing to the expansion of the pharmacovigilance market?
The escalation in research and development undertakings stimulates growth in the pharmacovigilance market. Pharmaceutical organizations can create novel and superior drugs through enhanced safety profiles by allocating resources to R&D. The intensive testing in preclinical and clinical stages during the drug development protocol allows early recognition of potential safety issues, paving the way for adequate risk reduction approaches.…
Monitoring Medication Safety with Pharmacovigilance
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. Pharmacovigilance plays a significant role in pharmaceutical and biotechnological sectors in designing of drugs and their interactions. The pharmacovigilance involves collecting information from healthcare providers and patients to know about the hazards associated with medications.
Download Sample PDF at: https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpnePR&utm_medium=10776
Increasing cases of adverse drug reactions…
Pharmacovigilance Market Opportunity Analysis by 2028
Pharmacovigilance Market: Introduction
According to the report, the global pharmacovigilance market was valued at US$ 6.1 Bn in 2020 and is projected to expand at a CAGR of 8.8% from 2021 to 2028. Pharmacovigilance activities are defined as science used for detection, assessment, understanding, and prevention of adverse effects of drugs and vaccines. Drugs and vaccines go through rigorous testing in the clinical trials to check their safety and efficacy before…