Press release
Clinical Trial Management System Market Poised for Significant Growth at 12.8% CAGR Through 2031 | Key Insights from Persistence Market Research
Overview of the MarketThe global Clinical Trial Management System (CTMS) market is witnessing significant growth, driven by the increasing complexity of clinical trials and the rise in the number of clinical studies conducted worldwide. Pharmaceutical and biotechnology companies are increasingly adopting CTMS solutions to streamline trial processes, improve regulatory compliance, and enhance the efficiency of their clinical operations.
CTMS solutions help manage various aspects of clinical trials, including patient recruitment, data collection, compliance tracking, and real-time monitoring, ensuring that the trials are conducted smoothly and efficiently.
Get a Sample PDF Brochure of the Report (Use Corporate Email ID for a Quick Response): https://www.persistencemarketresearch.com/samples/3017
The global CTMS market was valued at USD 846.9 million in 2024 and is expected to grow at a robust CAGR of 12.8%, reaching USD 2732.1 million by 2031. This growth can be attributed to several factors, including the rising demand for cloud-based and AI-driven solutions, which enable efficient remote management and data sharing across global teams. Furthermore, the shift toward decentralized and virtual trials has further amplified the need for flexible, scalable CTMS platforms.
North America is currently the leading geographical region in this market, owing to the presence of major pharmaceutical companies, increasing investments in research and development, and a favorable regulatory environment that encourages clinical trials and innovations.
Key Highlights from the Report
• The CTMS market is projected to grow from USD 846.9 million in 2024 to USD 2732.1 million by 2031.
• Cloud-based CTMS solutions are expected to be the leading segment due to their scalability, flexibility, and cost-effectiveness.
• North America holds the largest share of the market, accounting for nearly 48.9% of the global CTMS market in 2024.
• The pharmaceutical and biopharmaceutical industry is the largest end-user of CTMS, driving adoption for drug development and clinical trials.
• Web-based CTMS platforms are expected to dominate the market with an anticipated 56.7% share in 2024.
• The services segment is likely to grow at a CAGR of 13.3% due to the rising demand for comprehensive clinical trial management services.
Market Segmentation
By Product Type
The Clinical Trial Management System market is segmented by product type into cloud-based, web-based, and on-premises solutions. Cloud-based CTMS is the most preferred option, as it provides scalability, flexibility, and real-time data access, essential for managing trials across multiple locations. Cloud systems offer seamless integration with various digital tools, enabling global collaboration among research teams. As clinical trials become more complex and geographically dispersed, cloud-based solutions are increasingly seen as the best option due to their cost-effectiveness and ability to provide remote access. Web-based CTMS solutions are also gaining traction because of their scalability, cost-efficiency, and ease of use. On-premises solutions, though still in use, are less popular due to higher costs and less flexibility in handling large datasets.
By End-User
The CTMS market is also segmented by end-users, including pharmaceutical and biopharmaceutical companies, contract research organizations (CROs), and academic and research institutions. Pharmaceutical and biopharmaceutical companies are the largest end-users of CTMS solutions, as these organizations are responsible for the majority of clinical trials. The growing demand for new drugs, coupled with an increasing number of trials in therapeutic areas such as oncology, neurology, and infectious diseases, drives the adoption of CTMS platforms. CROs are also major users of CTMS, as they are often tasked with managing clinical trials for pharmaceutical companies. The need for efficient trial management solutions to ensure compliance, manage data, and improve collaboration across global sites further boosts the demand from CROs.
Regional Insights
North America
North America is the dominant region in the Clinical Trial Management System market. It is projected to account for nearly 48.9% of the global market share in 2024. The region's strong pharmaceutical and biotechnology industries, coupled with significant investments in research and development, are driving the adoption of CTMS. North America also benefits from a favorable regulatory environment, with the U.S. Food and Drug Administration (FDA) setting stringent guidelines that require clinical trials to be managed efficiently and in compliance with regulatory standards. Furthermore, the aging population in the region and the increasing prevalence of chronic diseases like diabetes, cardiovascular diseases, and cancer are fueling the demand for clinical trials and, by extension, CTMS solutions.
Asia Pacific
Asia Pacific is projected to experience the highest growth rate in the CTMS market due to the rapid increase in clinical trials and research activities in countries like China, India, and Japan. These countries are emerging as popular locations for clinical trials due to their large, diverse patient populations and lower costs. The growing investments in healthcare infrastructure and digital technologies are expected to drive the adoption of CTMS solutions in this region. Moreover, the increasing government focus on healthcare reforms and improving research capabilities further supports the expansion of the CTMS market in Asia Pacific.
Market Drivers
Growing Complexity of Clinical Trials
The increasing complexity of clinical trials is a key driver of the CTMS market. With the rise of multi-center trials, global collaborations, and the need for real-time data sharing across different time zones, traditional trial management methods are becoming insufficient. CTMS platforms offer centralized data management, improved patient tracking, and enhanced communication among stakeholders. This enables better decision-making, reduces the risk of errors, and accelerates trial timelines. The adoption of AI and machine learning in CTMS further enhances data accuracy and helps predict outcomes, improving trial efficiency.
Increasing Focus on Decentralized Clinical Trials
Another significant driver for the CTMS market is the increasing shift towards decentralized clinical trials (DCTs). These trials leverage digital tools, such as wearable devices and mobile applications, to monitor participants remotely and collect data in real-time. As DCTs gain popularity due to their flexibility and accessibility, there is a growing need for cloud-based CTMS platforms that can facilitate remote collaboration and data sharing. DCTs offer numerous benefits, including reduced patient burden, improved recruitment rates, and accelerated timelines, making them an attractive option for pharmaceutical companies and CROs.
Rising Demand for Personalized Medicine
The growing focus on personalized medicine is driving demand for more specialized clinical trials. As precision medicine continues to gain traction, clinical trials are becoming more individualized, targeting specific patient populations. CTMS solutions are well-suited for managing these complex trials by enabling better patient recruitment, improving trial monitoring, and enhancing data analysis. The ability to handle vast amounts of patient data and provide real-time insights into trial progress is essential for the success of personalized medicine trials, making CTMS a critical component of modern drug development.
Market Restraints
High Implementation Costs
While CTMS platforms offer numerous benefits, their high implementation and maintenance costs can be a barrier for smaller organizations, particularly in developing regions. The initial investment required for a CTMS system, including software, hardware, and training costs, can be prohibitive. Additionally, ongoing maintenance, updates, and support costs add to the financial burden. As a result, many small and medium-sized enterprises (SMEs) may be reluctant to adopt CTMS, limiting the overall market potential.
Data Security and Privacy Concerns
Data security and privacy remain significant concerns in the CTMS market, especially with the growing reliance on cloud-based systems. Clinical trial data, which includes sensitive patient information, must be protected against cyber threats and unauthorized access. The lack of standardized regulations across different countries regarding data privacy and security can create challenges for organizations seeking to comply with local laws and international standards. This uncertainty may deter some companies from adopting cloud-based CTMS solutions, particularly in regions with stringent data protection laws.
Market Opportunities
Cloud-Based Solutions
Cloud-based CTMS solutions present a significant opportunity for market growth. These platforms offer flexibility, scalability, and cost-effectiveness, making them ideal for managing the increasing volume and complexity of clinical trials. The ability to access data in real-time from any location allows for better collaboration among global teams and improved trial efficiency. As the demand for decentralized clinical trials grows, cloud-based solutions will become even more critical in ensuring seamless data sharing and management across different stakeholders.
Integration with AI and Analytics
The integration of AI and analytics into CTMS platforms offers a unique opportunity to enhance clinical trial management. AI can optimize trial design, participant recruitment, and retention strategies, while predictive analytics can improve decision-making and identify potential risks early in the trial process. By leveraging these technologies, CTMS platforms can streamline operations, reduce costs, and accelerate timelines, providing significant value to pharmaceutical companies, CROs, and other stakeholders in the clinical trial ecosystem.
Reasons to Buy the Report
✔ Comprehensive analysis of market trends, growth drivers, and opportunities
✔ Detailed segmentation of the market by product type, end-user, and geography
✔ Insights into the competitive landscape and key players operating in the market
✔ Forecasts on market growth and trends from 2024 to 2031
✔ In-depth analysis of regional trends, including North America, Asia Pacific, and Europe
Frequently Asked Questions (FAQs)
How Big is the Clinical Trial Management System Market?
Who are the Key Players in the Global Market for Clinical Trial Management Systems?
What is the Projected Growth Rate of the Clinical Trial Management System Market?
What is the Market Forecast for Clinical Trial Management Systems in 2032?
Which Region is Estimated to Dominate the Industry through the Forecast Period?
Company Insights
Key players in the Clinical Trial Management System market include:
Oracle Corporation
Medidata Solutions, Inc.
Veeva Systems
Parexel International Corporation
IBM Watson Health
Recent Developments
Medidata Solutions was recognized as a leader in the Everest Group's 2024 Life Sciences Clinical Trial Management System (CTMS) Products PEAK Matrix® Assessment.
Oracle announced enhancements to its Clinical One Randomization and Trial Supply Management (RTSM) system in May 2024, aimed at improving trial efficiency and adaptability.
Conclusion
The Clinical Trial Management System market is poised for substantial growth over the coming years, driven by the increasing complexity of clinical trials, the rise of decentralized trials, and the growing demand for cloud-based and AI-driven solutions. As the healthcare industry continues to prioritize efficiency, regulatory compliance, and data security,
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web:
https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Management System Market Poised for Significant Growth at 12.8% CAGR Through 2031 | Key Insights from Persistence Market Research here
News-ID: 3924061 • Views: …
More Releases from Persistence Market Research

Nickel-Metal Hydride Battery Market Predicted to See Expansion to US$ 4.9 Billio …
According to the latest study by Persistence Market Research, the global Nickel-Metal Hydride Battery Market is expected to be valued at US$ 3.6 billion in 2026 and is projected to reach US$ 4.9 billion by 2033, growing at a CAGR of 4.5% between 2026 and 2033. Despite the rapid rise of lithium-ion technologies, NiMH batteries continue to maintain strong relevance due to their safety profile, long cycle life, and established…
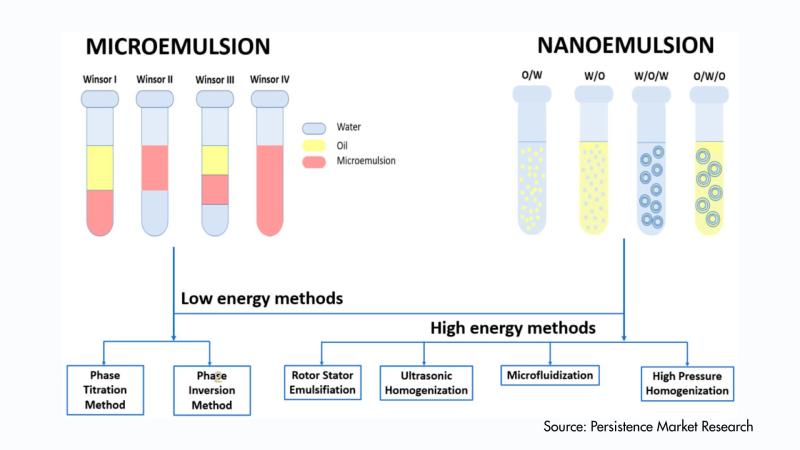
Microemulsion Market Poised for Growth to US$ 2.8 Billion by 2033, Driven by Exp …
According to the latest study by Persistence Market Research, the global microemulsion market is likely to be valued at US$ 1.8 billion in 2026 and is expected to reach US$ 2.8 billion by 2033, growing at a CAGR of 6.5% during the forecast period from 2026 to 2033. This steady growth reflects the rising demand for advanced formulation technologies across multiple industries, including pharmaceuticals, personal care, food & beverages, and…
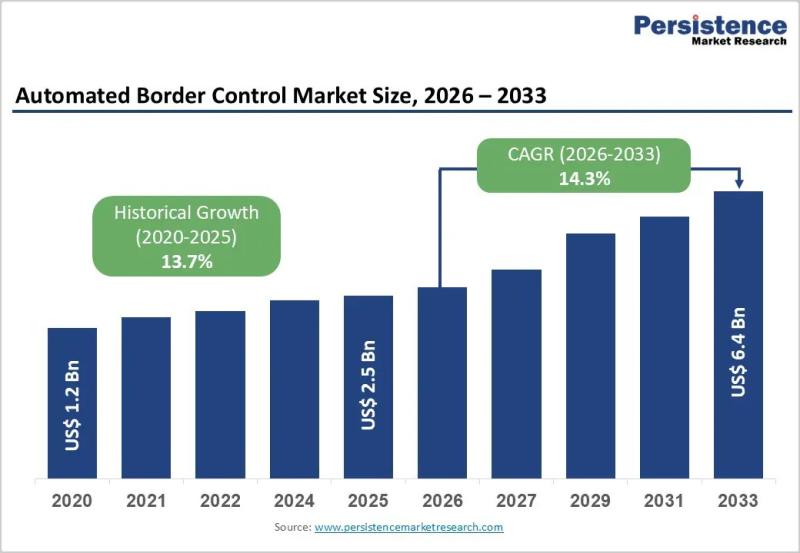
Automated Border Control Market Accelerates as Smart Borders Redefine Global Tra …
The automated border control (ABC) market has become a critical pillar of modern border management as governments worldwide respond to rising international travel volumes, security challenges, and the need for faster passenger processing. Automated border control systems use advanced biometric technologies-such as facial recognition, fingerprint scanning, and iris recognition-to verify traveler identities with minimal human intervention. These systems are most commonly deployed through ABC e-gates at airports, but their adoption…

Aluminum Composite Panels Market Set to Grow to US$14.0 Billion by 2033 Driven b …
The Aluminum Composite Panels Market is witnessing steady expansion as global construction activity accelerates and demand rises for lightweight, durable, and visually appealing building materials. According to the latest study by Persistence Market Research, the global aluminum composite panels market size is likely to be valued at US$ 9.1 billion in 2026 and is expected to reach US$ 14.0 billion by 2033, growing at a CAGR of 6.3% during the…
More Releases for CTMS
Key Trends Shaping the Future Clinical Trial Management System CTMS Market From …
What Is the Estimated Market Size and Growth Rate for the Clinical Trial Management System CTMS Market?
The clinical trial management system (CTMS) market has experienced rapid growth in recent years. It is expected to grow from $1.41 billion in 2024 to $1.61 billion in 2025, with a CAGR of 14.2%. Growth factors include increasing clinical trial complexity, a rise in global clinical trials, regulatory compliance demands, an increasing focus on…
Clinical Trial Management System (CTMS) Market: Growth, Trends, Opportunities, a …
Introduction
A Clinical Trial Management System (CTMS) is an integrated software platform designed to streamline and manage the planning, execution, and monitoring of clinical trials. It helps healthcare organizations, pharmaceutical companies, research institutions, and contract research organizations (CROs) efficiently oversee the complex and data-driven processes of clinical trials. The CTMS enables management of trial activities such as patient recruitment, data collection, regulatory compliance, budgeting, and reporting.
Clinical trials are a critical component…
Clinical Trial Management System (CTMS) Market: Growth, Opportunities, and Chall …
Introduction
The Clinical Trial Management System (CTMS) market is a crucial segment of the global healthcare and pharmaceutical industries, which supports the efficient and accurate management of clinical trials. CTMS refers to software systems used by pharmaceutical, biotechnology, and contract research organizations (CROs) to streamline the planning, tracking, and management of clinical trials. These systems provide centralized data management for clinical trial activities, which helps improve the speed, quality, and compliance…
Clinical Trial Management System (CTMS) Global Market Report 2024 - Clinical Tri …
"The Business Research Company recently released a comprehensive report on the Global Clinical Trial Management System (CTMS) Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
According to The Business Research Company's, The…
Clinical Trial Management System (CTMS) Market Scope & Future Opportunities Till …
An exclusive Clinical Trial Management System (CTMS) Market research report has been fabricated through the in depth analysis of the market dynamics across five regions including North America, Europe, South America, Asia-Pacific, Middle East and Africa. The segmentation of the market by components, end users, and region was done based on the thorough market analysis and validation through extensive primary inputs from industry experts (key opinion leaders of companies, and…
Latest Clinical Trial (CTMS) Market 2022 | Detailed Report
ReportsnReports publishes the report titled Clinical Trial (CTMS) that presents a 360-degree overview of the market under one roof. The report is developed with the meticulous efforts of an enthusiastic and experienced team of experts, analyts, and researchers that makes the report a valuable asset for stakeholders to make robust decisions. This report also provides an in-depth overview of product type, specification, technology, and production analysis considering vital factors like…
