Press release
🚀 The Promising Future of Cell and Gene Therapy (CGT): Transforming Healthcare 🚀
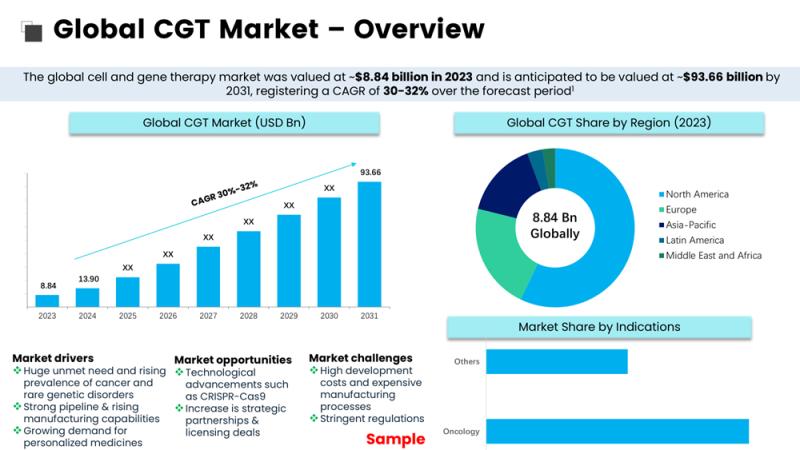
Cell and Gene Therapy (CGT) is one of modern medicine's most rapidly advancing and transformative fields. By offering groundbreaking treatments that address the root causes of genetic disorders, cancers, and other complex diseases, CGT is reshaping healthcare and providing new hope for patients worldwide. With continuous advancements in genetic engineering, stem cell research, and immunotherapy, CGT is paving the way for personalized healthcare and potential cures for previously untreatable conditions.What is Cell and Gene Therapy?
Gene therapy involves modifying or replacing defective genes within a patient's cells to treat or prevent disease. This can be achieved by inserting, deleting, or altering genetic material. Gene therapy is being researched for conditions like cystic fibrosis, sickle cell anemia, and muscular dystrophy.
Cell therapy uses living cells to repair or replace damaged tissues or organs. Stem cell therapy is the most widely known form of cell: therapy, aimed at regenerating tissue damaged by disease or injury. Another highly innovative application is CAR-T (chimeric antigen receptor T-cell) therapy, which modifies immune cells to target and destroy cancer cells.
Together, gene and cell therapies combine to address diseases at a fundamental level-by correcting faulty genes or introducing new cells to restore the body's functionality. This has the potential to transform the way we approach medical treatment.
For More detailed Competitive Intelligence insights on Cell and Gene Therapy (CGT) : https://www.datamintelligence.com/competitive-intelligence
How Does CGT Work?
1. Gene Editing and Replacement:
o Gene Editing: Tools like CRISPR-Cas9 enable precise modifications to a patient's DNA, allowing scientists to correct mutations at the genetic level. In 2022, researchers used CRISPR technology to treat patients with sickle cell anemia, marking a landmark achievement in gene therapy. This technique helped these patients produce healthy red blood cells after receiving the edited stem cells, with results lasting for years.
o Gene Replacement: For conditions caused by missing or defective genes, such as spinal muscular atrophy (SMA), a healthy copy of the gene can be inserted into the patient's cells. In 2020, Zolgensma was approved by the FDA as the first gene therapy for SMA, with a price tag of $2.1 million per dose. Zolgensma has shown life-changing results, significantly improving motor skills in children diagnosed with this rare genetic disorder.
2. Stem Cell Therapy:
o Stem cells are undifferentiated cells capable of developing into specialized cells. When injected into the body, they can regenerate damaged tissues or organs. In 2019, Prochymal, a stem cell-based therapy, was used to treat patients with graft-versus-host disease (GvHD), showing significant improvements in patient outcomes, with reduced mortality rates.
3. Immunotherapy:
o CAR-T Therapy involves genetically modifying a patient's T cells to express receptors that recognize and attack cancer cells. CAR-T therapies like Kymriah (Novartis) and Yescarta (Gilead) have revolutionized treatment for blood cancers like leukemia and lymphoma. In 2023, Yescarta became the first CAR-T therapy to be approved for treating adult patients with relapsed or refractory follicular lymphoma, a type of non-Hodgkin lymphoma, further expanding the scope of CAR-T's impact on cancer treatment.
Applications of CGT
The applications of CGT are vast, ranging from rare genetic disorders to complex diseases like cancer. The recent data highlights the growing impact of CGT in several medical fields:
• Genetic Disorders: Gene therapies are showing remarkable success in treating rare genetic diseases. For instance, Zolgensma for SMA is leading the charge, with over 1,000 children treated globally. In 2023, Bluebird Bio announced that its gene therapy for sickle cell disease, Zynteglo, successfully cured patients who had not responded to traditional treatments, offering a life-changing alternative for those with severe cases.
• Cancer Treatment: The FDA has approved several CAR-T therapies for blood cancers. Kymriah, approved in 2017, was the first CAR-T treatment to receive FDA approval, and Yescarta, approved in 2017, has been particularly successful in treating large B-cell lymphoma. A 2024 study showed that Yescarta has an overall response rate of 80% in patients with refractory follicular lymphoma, underscoring the growing success of CAR-T therapies.
• Regenerative Medicine: Stem cell therapies are being tested to treat degenerative diseases, such as macular degeneration, heart disease, and spinal cord injuries. Prochymal, used in treating Crohn's disease and GvHD, has shown promise in improving symptoms and reducing long-term complications. Additionally, Astellas Pharma is advancing research on its stem cell-based therapies to repair heart tissue, with clinical trials expected to ramp up in 2025.
• Autoimmune Diseases: CGT is being explored as a way to modulate the immune system in autoimmune diseases like rheumatoid arthritis and lupus. Early studies in 2023 demonstrated the potential of gene editing to "reset" malfunctioning immune cells in autoimmune conditions, offering a promising avenue for treating diseases that have limited treatment options.
Regulatory Approvals and Clinical Trials
The regulatory landscape for CGT is rapidly evolving, with an increasing number of therapies gaining approval. According to the FDA, in 2024, the number of FDA-approved CGT treatments reached 24, and this number is expected to rise substantially in the coming years. Regulatory agencies across the globe, including the European Medicines Agency (EMA), are also working to streamline approval processes while ensuring patient safety.
Some of the most notable recent approvals include:
• Ryoncil (December 2024/Mesoblast): The US FDA approved Ryoncil® (remestemcel-L) as the first mesenchymal stromal cell therapy in the US for steroid-refractory acute graft versus host disease (SR-aGvHD) in children 2 months and older, including adolescents and teenagers.
• Kebilidi (November 2024/PTC Therapeutics,): The US FDA approved Kebilidi (eladocagene exuparvovecadeno-associated-tneq), an adeno-associated virus vector-based gene therapy indicated for the treatment of adult and pediatric patients with aromatic L-amino acid decarboxylase (AADC) deficiency.
• Aucatzyl (November 2024/Autolus Inc.): The US FDA approved obecabtagene autoleucel, a CD19-directed genetically modified autologous T cell immunotherapy, for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
Syndicate report: https://www.datamintelligence.com/research-report/cell-and-gene-therapy-market
🌍 Global Impact:
• As of 2024, more than 2,500+ clinical trials are underway, with CGT expanding into a wider range of diseases, from cancer to autoimmune disorders. This represents a more than 100% increase in the number of trials since 2018, illustrating the accelerating pace of innovation.
• FDA and EMA have approved several CGT treatments, and regulatory agencies are adapting guidelines to ensure patient safety and speed up approval processes.
Challenges and Considerations
Despite the promising progress, CGT still faces significant challenges:
1. Safety Concerns: Safety remains a top priority, particularly with gene editing and cell-based therapies. 2023 clinical data highlighted the risk of cytokine release syndrome (CRS) in CAR-T treatments, which can cause severe inflammation. Although advances in managing CRS have been made, ongoing research into improving safety and reducing side effects is crucial.
2. Regulatory Hurdles: The FDA and other global agencies have approved several CGT therapies, but the approval process remains complex, with each new therapy requiring extensive data on safety and efficacy. While the regulatory environment is improving, it still lags behind the speed of technological advancements.
3. Cost: The cost of CGT treatments remains a barrier for many patients. For example, Zolgensma costs $2.1 million per treatment, making it one of the most expensive drugs in the world. While the high cost reflects the complexity of these therapies, insurers and healthcare systems worldwide are grappling with how to manage these prices.
4. Ethical Considerations: The ethical implications of germline editing-altering DNA in eggs, sperm, or embryos-remain contentious. While somatic gene therapies (altering non-reproductive cells) are largely accepted, debates over germline editing continue to shape policies.
Get Free Updates: https://www.datamintelligence.com/contact
Mergers and Acquisitions: Fueling CGT Growth
The CGT space is witnessing rapid mergers and acquisitions as larger pharmaceutical companies acquire biotech firms to expand their portfolios in this area. These acquisitions fuel growth by consolidating resources and accelerating the development of innovative therapies.
• December 2024: Astellas and Sangamo Therapeutics entered into a license agreement allowing Astellas to leverage Sangamo's novel proprietary neurotropic adeno-associated virus (AAV) capsid, STAC-BBB, which has demonstrated potent blood-brain barrier penetration and neuronal transduction in nonhuman primates.
• December 2024: Amarna Therapeutics announced its strategic collaboration with NorthX Biologics to advance the clinical trial manufacturing of Nimvec AM510, Amarna's groundbreaking gene therapy platform designed to transform the treatment of immune-mediated diseases.
• November 2024: Roche entered into a definitive merger agreement to acquire Poseida Therapeutics, Inc. for US $9.00 per share in cash at closing, representing a total equity value of approximately US $1.0 billion. The transaction is expected to close in the first quarter of 2025. Poseida's R&D portfolio includes pre-clinical and clinical-stage off-the-shelf CAR-T therapies
The Future of CGT
The future of CGT looks incredibly promising as research continues to evolve. Key developments on the horizon include:
• Personalized Medicine: CGT is expected to become more personalized, with treatments tailored to individual genetic profiles for enhanced efficacy.
• Expansion Beyond Rare Diseases: CGT is expanding beyond rare genetic disorders and is being applied to more common conditions like cancer, heart disease, and diabetes.
• Improved Delivery Mechanisms: Advances in non-viral delivery systems and more efficient gene delivery methods are expected to improve the safety and effectiveness of CGT, making it more accessible to a broader range of patients.
• Global Access: Efforts are underway to lower the cost of CGT treatments, making them more accessible to people in low- and middle-income countries. 2025 may see new pricing models and insurance coverage options that facilitate wider access to these life-changing therapies.
Conclusion
Cell and Gene Therapy is revolutionizing the field of medicine, offering treatments that could cure genetic disorders, combat cancer, and regenerate damaged tissues. While challenges like safety, cost, and regulatory approval remain, recent data and advancements suggest that the future of CGT is incredibly bright. As clinical trials continue to show promise and new therapies gain regulatory approval, CGT has the potential to change millions of lives worldwide, making treatments that once seemed impossible a reality.
🌟 Looking Ahead: The future of CGT is promising, with advancements in personalized medicine, improved delivery systems, and expanded access for patients in low-income regions. As research progresses, CGT could become the norm for treating complex diseases, providing hope where there was once none.
💬 What do you think? How will CGT shape the future of medicine in the next decade?
Speak to our analyst:
Name: Mallikarjun Anne
Email: Mallikarjun@datamintelligence.com
#CellAndGeneTherapy #GeneticMedicine #CAR-T #Immunotherapy #HealthcareInnovation #PersonalizedMedicine #GeneEditing #CRISPR #RegenerativeMedicine #Pharma
DATAM INTELLIGENCE 4MARKET RESEARCH LLP
HABSIGUDA, IDA - UPPAL
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release 🚀 The Promising Future of Cell and Gene Therapy (CGT): Transforming Healthcare 🚀 here
News-ID: 3826981 • Views: …
More Releases from DataM Intelligence 4market Research LLP

Global Ride Sharing Market to Grow at 15.85% CAGR Through 2031 Driven by AI Inte …
The Global Ride Sharing Market is expected to grow at a CAGR of 15.85% during the forecast period (2024 - 2031).
Ride-sharing services connect passengers with drivers through digital platforms, offering convenient and cost-effective transportation solutions. Companies like Ub*r, and Grab dominate the market, providing options such as carpooling, electric vehicles, and premium rides. The industry is driven by urbanization, changing consumer preferences, and environmental concerns. Integration of AI, route optimization,…
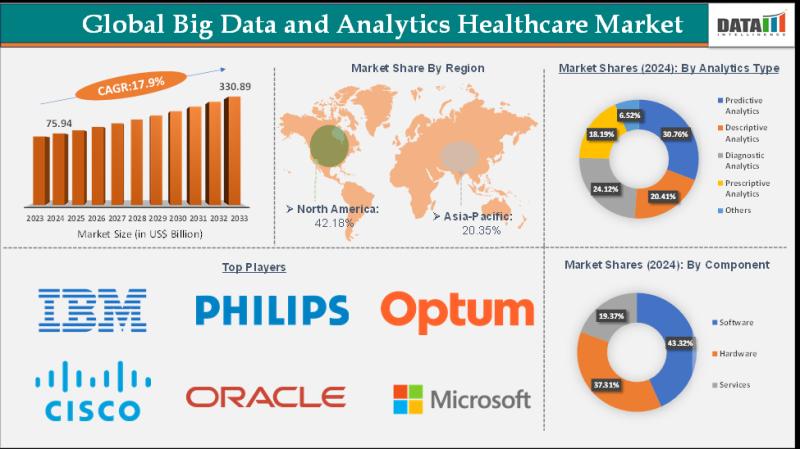
Global Big Data and Analytics Healthcare Market to Reach US$ 330.89 Billion by 2 …
Big Data and Analytics Healthcare Market Size and Forecast
The global big data and analytics healthcare market size reached US$ 75.94 Billion in 2024 and is expected to reach US$ 330.89 Billion by 2033, growing at a CAGR of 17.9% during the forecast period 2025-2033.
DataM Intelligence has published a new research report on "Big Data and Analytics Healthcare Market Size 2025". The report explores comprehensive and insightful Information about various key…
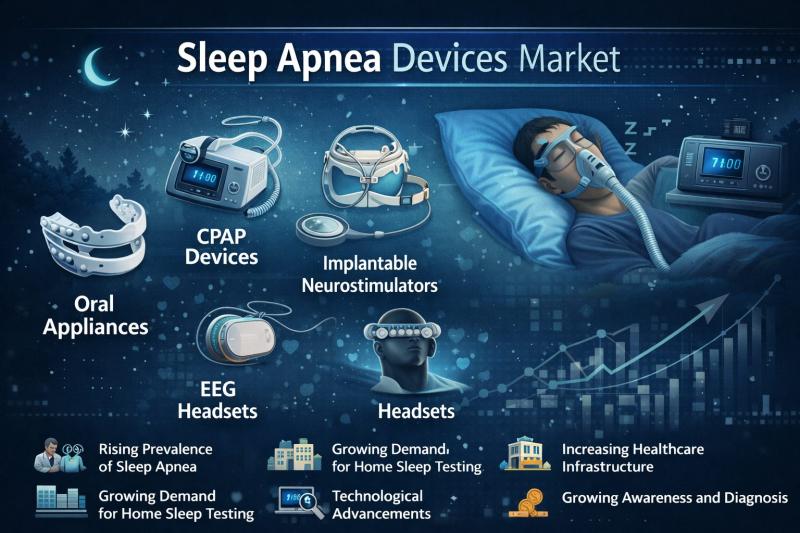
Japan Sleep Apnea Devices Market to Expand at 7.90% CAGR Through 2031 Driven by …
Sleep Apnea Devices Market size was valued at USD 7,981.80 million in 2021 and is estimated to reach at a compound annual growth rate (CAGR) of 7.90% over the forecast period 2024-2031.
Sleep apnea devices include CPAP machines, BiPAP devices, and oral appliances designed to manage sleep apnea by ensuring proper airflow during sleep. These devices help reduce snoring, improve oxygen levels, and enhance sleep quality. The market is driven by…
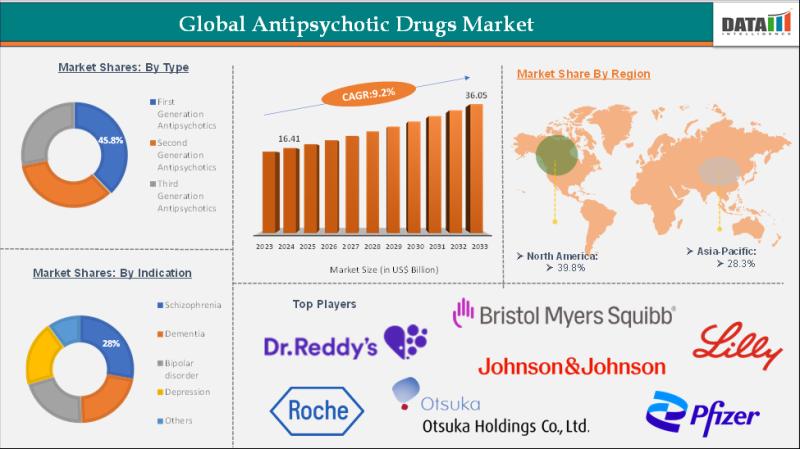
Antipsychotic Drugs Market Size to Reach US$ 32.83 Billion by 2032 at 7.9% CAGR …
Antipsychotic Drugs Market Size and Forecast
The Global Antipsychotic Drugs Market reached US$ 16.41 billion in 2024 and is expected to reach US$ 32.83 billion by 2032, growing at a CAGR of 7.9% during the forecast period 2025-2032.
DataM Intelligence has published a new research report on "Antipsychotic Drugs Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of…
More Releases for CGT
Graphite Felt & Carbon Felt Market to Witness Remarkable Growth by 2030 | CGT Ca …
The Latest Released Graphite Felt & Carbon Felt market study has evaluated the future growth potential of Global Graphite Felt & Carbon Felt market and provides information and useful stats on market structure and size. The report is intended to provide market intelligence and strategic insights to help decision-makers take sound investment decisions and identify potential gaps and growth opportunities. Additionally, the report also identifies and analyses changing dynamics, and…
Battery Felts Market Business Opportunities 2026 - Top Companies are Mersen,SGL …
A detailed research study on the Battery Felts Market was recently published by DataIntelo. The report puts together a concise analysis of the growth factors influencing the current business scenario across various regions. Significant information pertaining to the industry analysis size, share, application, and statistics are summed in the report in order to present an ensemble prediction. Additionally, this report encompasses an accurate competitive analysis of major market players and…
Graphite Felts Market Business Opportunities 2026 - Top Companies are Mersen,SGL …
The Graphite Felts Market report includes overview, which interprets value chain structure, industrial environment, regional analysis, applications, market size, and forecast. The report provides an overall analysis of the market based on types, applications, regions, and for the forecast period from 2020 to 2026. It also offers investment opportunities and probable threats in the market based on an intelligent analysis.
This report focuses on the Global Graphite Felts Market trends, future…
Automotive Artificial Leather Market Business Opportunities 2026 - Top Companies …
Automotive Artificial Leather Market Forecast 2020-2026
The Global Automotive Artificial Leather Market research report provides and in-depth analysis on industry- and economy-wide database for business management that could potentially offer development and profitability for players in this market. It offers critical information pertaining to the current and future growth of the market. It focuses on technologies, volume, and materials in, and in-depth analysis of the market. The study has a section…
Global PVC Artificial Leather Market Report 2019 Companies included CGT, Vulcafl …
Market Reports Company has recently published 7th edition of PVC Artificial Leather Market Report which covers historic data of year 2013 to 2018 along with a forecast till year 2026.
For inquiry or free sample pages email us at: sales@marketreportscompany.com
The report provides a comprehensive view on the current state and future prospects of the market which analyzes different strategies for business growth. The global PVC Artificial Leather market was valued at…
Global Coated Fabrics Market 2019 - OMNOVA Solutions, Takata(Highland Industries …
This new report by Eon Market Research, titled “Global Coated Fabrics Market 2019 Research Report, 2015 – 2025” offers a comprehensive analysis of Coated Fabrics industry at a global as well as regional and country level. Key facts analyzed in this report include the Coated Fabrics market size by players, regions, product types and end industries, history data 2014-2018 and forecast data 2019-2025. This report primarily focuses…