Press release
RNAi Therapeutics Market Advancements in Gene Therapy and Its Impact on Healthcare
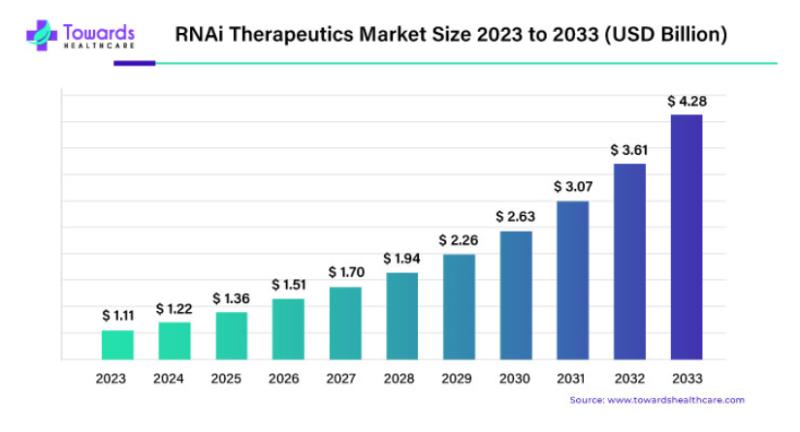
In recent years, the RNA interference (RNAi) therapeutics market has experienced a remarkable transformation. What was once a specialized and relatively niche sector has rapidly emerged as one of the most promising areas of biomedical innovation. As of 2023, the RNAi therapeutics market was valued at an impressive USD 1.11 billion, marking a significant milestone in the industry's expansion. However, this is just the beginning. With the market poised for an astonishing 14.9% compound annual growth rate (CAGR) over the next decade, industry experts predict that by 2033, the market could surge to a staggering USD 4.28 billion.Download Statistical Data: https://www.towardshealthcare.com/download-statistics/5115
siRNA Therapy: Leading the Charge
A driving force behind the market's rapid growth is small interfering RNA (siRNA) therapy, which is expected to maintain its dominant position in the coming years. In 2023, siRNA-based therapies accounted for an overwhelming 64% of the RNAi therapeutics market share. These therapies work by silencing specific genes, thus offering the potential to treat a wide array of diseases previously considered untreatable. As advancements in RNAi technology continue to progress, siRNA therapies are being increasingly recognized for their ability to target the underlying genetic causes of diseases, offering hope to patients with conditions ranging from genetic disorders to cancer.
Oncology: The Prime Target
One of the most promising applications of RNAi therapeutics lies in oncology. Cancer continues to be the dominant therapeutic target in the RNAi space, with oncology treatments expected to maintain their leadership throughout the forecast period. The ability to target cancer cells with greater precision and specificity is a breakthrough that RNAi therapies are uniquely positioned to provide. By silencing the genes that contribute to cancer cell growth, RNAi-based treatments could revolutionize how oncologists approach treatment, providing more effective options and fewer side effects compared to conventional therapies.
The shift toward targeted cancer treatments, combined with the rapid pace of research and clinical trials, is accelerating the adoption of RNAi therapeutics in the oncology field. As the understanding of cancer's genetic underpinnings deepens, the role of RNAi in developing personalized and highly effective cancer therapies is becoming more pronounced.
North America: A Market Leader
North America is leading the charge in the RNAi therapeutics market, holding a commanding 41% share of the market in 2023. The region benefits from a well-established healthcare infrastructure, robust research and development capabilities, and strong investments from both public and private sectors. The United States, in particular, is home to numerous biotech companies at the forefront of RNAi research, with several therapies already in advanced clinical stages.
In addition to the market's strong presence in North America, the increasing demand for precision medicine and advancements in RNAi technologies are expected to drive further growth in the region. The U.S. Food and Drug Administration (FDA) has also played a crucial role in expediting the approval process for RNAi therapies, which is expected to further bolster market expansion.
A Bright Future for RNAi Therapeutics
The future of RNAi therapeutics looks incredibly promising, with innovations continuing to unfold at a rapid pace. As the market grows, new applications for RNAi are emerging, expanding its potential beyond oncology to include conditions like cardiovascular diseases, genetic disorders, and viral infections. The ongoing development of novel delivery mechanisms and improvements in the stability and specificity of RNAi molecules are crucial factors that will support the widespread adoption of these therapies.
As the industry prepares for its next phase of growth, stakeholders in the pharmaceutical and biotech sectors are keenly aware of the potential that RNAi therapeutics hold for transforming the treatment landscape. With the market set to reach USD 4.28 billion by 2033, the RNAi therapeutics space is one to watch closely in the coming years. Whether it's through breakthroughs in cancer treatment or the emergence of new therapeutic applications, RNAi is undoubtedly at the forefront of the next generation of medical innovation.
Rising Trends and Shifts in RNAi Therapeutics: Paving the Way for Revolutionary Treatments
RNA interference (RNAi) therapeutics have undergone a significant evolution since their discovery in the early 1990s, making an even greater impact in the world of medicine after the advent of clinical trials in 2008. Initially a promising but specialized field, RNAi technology has now emerged as one of the most revolutionary approaches to treating genetic disorders and diseases such as cancer, providing hope for a wide range of patients. The journey of RNAi therapeutics, from laboratory research to clinical trials, is one marked by significant milestones, challenges, and a transformative potential for medical science.
A Surge in RNAi Clinical Trials
The landscape of RNAi therapeutics has undergone remarkable change over the past decade, with clinical trials serving as the critical platform for demonstrating the potential of these innovative treatments. From 2008 to 2022, the number of clinical trials incorporating RNAi-based therapies grew nearly tenfold, a testament to the increasing interest and investment in the technology. During this period, the number of ongoing trials soared by about sevenfold, with RNAi-based therapeutics being tested in a diverse array of diseases.
However, the RNAi trial landscape experienced a noticeable shift in 2022, with a significant 40% drop in ongoing studies involving small interfering RNA (siRNA) drugs. Despite this decline, siRNAs continue to dominate the RNAi therapeutic field. In 2022, nearly 90% of both published and ongoing clinical trials focused on siRNA-based drugs, reinforcing their central role in advancing RNAi therapeutics. This trend has continued into 2023, where the overwhelming majority of published and ongoing trials are centered on siRNA, which remains the most widely used RNAi molecule in clinical research.
On the other hand, research involving short hairpin RNAs (shRNAs) has remained limited but steady, with shRNA-based treatments appearing in a small yet consistent number of studies over the years. Similarly, the investigation of microRNAs (miRNAs) and miR-shRNAs has remained relatively niche, with fewer trials exploring these molecules.
The Rising Incidence of Cancer and the Role of RNAi in Treatment
Cancer continues to be one of the most pressing global health challenges, with the American Cancer Society estimating that nearly 1.92 million new cancer cases and 609,360 cancer-related deaths occurred in the United States alone in 2022. This global health crisis underscores the urgent need for novel and effective treatments. RNA interference technology has surfaced as a powerful tool in the fight against cancer, offering new hope for patients battling this devastating disease.
RNAi works by targeting specific genes involved in cancer growth, effectively silencing the genes that contribute to the uncontrolled cell division characteristic of cancer. By leveraging this natural biological process, RNAi-based therapeutics can offer a more precise, targeted approach to cancer treatment, setting them apart from traditional therapies like chemotherapy that often harm both cancerous and healthy cells. This targeting capability has the potential to minimize side effects and improve treatment outcomes.
A significant example of RNAi's potential in cancer treatment can be seen in the November 2023 approval of Givlaari (givosiran), developed by Alnylam Pharmaceuticals. While Givlaari is not a direct cancer treatment, it illustrates the capabilities of RNAi-based therapies in treating genetic diseases, such as acute hepatic porphyria (AHP). AHP is a metabolic disorder caused by a specific genetic syndrome, and Givlaari targets the liver X gene involved in the disease's progression. This treatment highlights the broader potential of RNAi therapeutics, including their use in rare genetic diseases and complex conditions like cancer.
The application of RNAi therapeutics in oncology is poised for growth, as scientists continue to refine their understanding of the genetic mechanisms underlying various cancers. Researchers and pharmaceutical companies are investing heavily in developing RNAi-based treatments, with the hope that they will transform the treatment landscape for cancer patients.
Progress in RNAi Clinical Trials and Preclinical Studies: A Glimpse into the Future
RNAi therapeutics are progressing through multiple phases of development, from preclinical research to clinical trials, with each stage offering critical insights into the safety and efficacy of these treatments.
The preclinical phase is essential for testing RNAi technology in controlled laboratory settings and animal models. During this stage, scientists work to understand the mechanisms of RNAi, craft RNAi molecules, and evaluate their effectiveness and safety. Preclinical studies have been pivotal in demonstrating the potential of RNAi therapeutics, sparking increased interest and investment in the technology. As these studies show promising results, they pave the way for further exploration and advancement in clinical trials.
Once preclinical research yields positive outcomes, RNAi therapeutics move into clinical trials, where they are tested in human subjects to assess their safety, efficacy, and tolerability. These trials are conducted in phases, starting with Phase I, which focuses on safety, followed by Phase II, where efficacy is evaluated, and finally, Phase III, which involves large-scale studies to confirm the therapeutic's effectiveness and safety. Positive results in clinical trials can propel RNAi-based therapies toward commercialization and regulatory approval.
The continuous success of RNAi therapeutics in clinical trials is bolstered by the increasing investment and partnerships between pharmaceutical companies, biotech firms, and research institutions. These collaborations are driving forward the development of RNAi-based treatments across various therapeutic areas, from oncology to rare genetic disorders, and are laying the groundwork for the next generation of breakthrough treatments.
The Future of RNAi Therapeutics
The RNAi therapeutics market is at a crossroads, poised for dramatic growth. The increased prevalence of diseases such as cancer and genetic disorders has fueled a rising demand for more effective and targeted therapies. RNAi, with its precision and specificity, is positioned to meet this demand.
The future of RNAi therapeutics looks incredibly promising, with advancements in delivery technologies, molecule stability, and gene targeting continuing to improve. As researchers refine RNAi molecules and explore their application in a wider range of diseases, the potential for RNAi to revolutionize the treatment of a variety of medical conditions is vast.
Investors and pharmaceutical companies are keenly aware of the potential RNAi therapeutics hold, and their growing interest in this space is expected to accelerate the development of new RNAi-based treatments. With an increasing number of trials and successful clinical outcomes, the RNAi market is on track for continued expansion, offering new hope to patients worldwide.
Groundbreaking Developments in RNA-based Therapeutics: A New Era of Treatment
The realm of RNA-based therapeutics has been experiencing rapid and revolutionary advancements, with notable breakthroughs coming from leading pharmaceutical companies and researchers. These cutting-edge therapies are not only providing new hope for patients battling life-threatening conditions, but they also mark a significant leap forward in the medical field. Two major milestones in 2022 and 2023 exemplify this surge of progress: Moderna's breakthrough mRNA vaccine and Alnylam Pharmaceuticals' approval for RNAi therapy.
Moderna's mRNA Vaccine: A Game-Changer for Respiratory Disease Prevention
In January 2023, the U.S. Food and Drug Administration (FDA) officially granted Breakthrough Therapy designation to Moderna's new mRNA vaccine, mRNA-1345. This prestigious recognition highlights the vaccine's potential to make a substantial impact on public health, particularly in protecting vulnerable populations from respiratory diseases.
The vaccine targets respiratory syncytial virus (RSV), a significant cause of respiratory infections in older adults. RSV infection is a leading contributor to hospitalization and severe respiratory illness, especially in individuals over the age of 60. Given the global rise in elderly populations, the need for effective preventive measures against such infections has never been more critical.
Moderna's mRNA-1345 vaccine is designed to stimulate the body's immune system to defend against RSV by introducing a small fragment of the virus's genetic material (mRNA), triggering the production of protective antibodies. This breakthrough is not only an important step forward in the fight against RSV but also underscores the versatility and potential of mRNA technology to address a wide array of infectious diseases. The success of this vaccine could pave the way for future vaccines targeting other respiratory viruses and pathogens, offering renewed hope for older adults at risk of severe respiratory conditions.
Alnylam Pharmaceuticals' RNAi Therapy for Hereditary Disease
Meanwhile, in September 2022, Alnylam Pharmaceuticals, a leader in RNA interference (RNAi) therapeutics, made significant strides with the European Commission's approval of AMVUTTRA, an innovative RNAi-based treatment. AMVUTTRA is specifically designed to treat adults with hereditary transthyretin-mediated (hATTR) polyneuropathy in stages 1 or 2-a rare and progressive genetic disorder.
hATTR polyneuropathy is caused by the accumulation of abnormal transthyretin (TTR) proteins in the peripheral nervous system, leading to nerve damage and potentially debilitating symptoms such as muscle weakness, pain, and impaired organ function. This condition often goes undiagnosed until it reaches advanced stages, and there have been few effective treatments to address its underlying cause.
AMVUTTRA works by targeting and silencing the gene responsible for producing the abnormal TTR protein. By inhibiting the production of the protein, the drug prevents the buildup of harmful aggregates that cause nerve damage. The approval of AMVUTTRA offers a new lease on life for individuals suffering from this debilitating disorder, providing a targeted approach that addresses the root cause of the disease rather than merely alleviating symptoms.
Alnylam's approval by the European Commission represents a significant milestone in the field of RNAi therapeutics. It validates the power of RNA-based technologies in treating complex genetic disorders, opening up new possibilities for developing treatments for other rare and genetically driven diseases. This approval further strengthens the case for RNAi therapy as a promising solution for many intractable conditions, which could eventually lead to the development of other targeted treatments.
The Future of RNA-based Therapies
These recent developments in mRNA and RNAi therapeutics signal a profound shift in the way we approach medical treatment. mRNA technology, propelled by its success in COVID-19 vaccines, is now being harnessed for a broader range of respiratory infections. Meanwhile, RNAi therapies, like AMVUTTRA, demonstrate the power of genetic silencing to address the underlying causes of hereditary and degenerative diseases. Together, these innovations are changing the landscape of medicine, promising a future where treatments are not only more effective but also more personalized and targeted.
As the world continues to grapple with an aging population and increasing prevalence of chronic diseases, the approval of these therapies represents a critical step forward. Moderna's mRNA vaccine offers hope for combating serious respiratory diseases in elderly populations, while Alnylam's RNAi therapy gives patients suffering from rare, genetically driven disorders a fighting chance against debilitating conditions.
Source: https://www.towardshealthcare.com/insights/rnai-therapeutics-market-sizing
Baner
Buy Premium Global Insight: https://www.towardshealthcare.com/price/5115
Review the Full TOC for the RNAi Therapeutics Market Report: https://www.towardshealthcare.com/table-of-content/rnai-therapeutics-market-sizing
Get the latest insights on industry segmentation with our Annual Membership https://www.towardshealthcare.com/get-an-annual-membership
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
We've prepared a service to support you. Please feel free to contact us at sales@towardshealthcare.com
Web: https://www.towardshealthcare.com
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release RNAi Therapeutics Market Advancements in Gene Therapy and Its Impact on Healthcare here
News-ID: 3811785 • Views: …
More Releases from Towards Healthcare

Rapid Advancements in Cardiac Biomarkers Shaping the Future of Healthcare
The global cardiac biomarkers market is experiencing substantial growth, driven by a surge in the number of cardiovascular diseases (CVDs) and a rising demand for early diagnostics and preventive care. Valued at USD 21.27 billion in 2024, the market is projected to grow to USD 24.39 billion in 2025 and reach an impressive USD 83.54 billion by 2034, expanding at a compound annual growth rate (CAGR) of 14.66% during this…

Rapid Advancements in Cardiac Biomarkers Shaping the Future of Healthcare
The global cardiac biomarkers market is experiencing substantial growth, driven by a surge in the number of cardiovascular diseases (CVDs) and a rising demand for early diagnostics and preventive care. Valued at USD 21.27 billion in 2024, the market is projected to grow to USD 24.39 billion in 2025 and reach an impressive USD 83.54 billion by 2034, expanding at a compound annual growth rate (CAGR) of 14.66% during this…

Exploring the Impact of Robotics on the Dental Industry
The field of robotic dentistry is rapidly evolving, with technological advancements and the increasing prevalence of dental diseases driving substantial growth. Valued at an estimated US$ 535 million in 2023, the robotic dentistry market is poised to reach US$ 2.58 billion by 2034, growing at an impressive compound annual growth rate (CAGR) of 15.4% from 2024 to 2034. This surge is driven by a combination of factors, including innovation in…

Revolutionizing Industries with Key Developments in the Microbial Fermentation T …
The microbial fermentation technology market is rapidly expanding, reflecting a broader shift towards sustainable and bio-based manufacturing processes. Valued at approximately USD 34.11 billion in 2023, the market is set to experience significant growth, with projections placing its value at USD 60.17 billion by 2033. This growth is anticipated at a compound annual growth rate (CAGR) of 5.84% from 2024 to 2033. The rise in demand for biologics, coupled with…
More Releases for RNA
CD Formulation Launches Custom Circular RNA Synthesis Service to Accelerate RNA …
CD Formulation introduces a customizable circRNA synthesis service, delivering high-quality, stable circRNAs for therapeutics, vaccines, and gene research, supported by advanced design and QC processes.
CD Formulation, a leading provider of advanced small nucleic acid synthesis [https://www.formulationbio.com/nucleic-acid/custom-small-nucleic-acid-synthesis.html] solutions, is proud to announce the launch of its fully customizable circular RNA (circRNA) synthesis service. This new service addresses the growing need for stable, non-immunogenic RNA molecules for therapeutic development, vaccine research, and…
Self-Amplifying RNA Synthesis Market Gains Traction as Biotech Firms Embrace Sca …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " Self-Amplifying RNA Synthesis Market- (By Product & Service (Products (Enzymes & Reagents, Premade saRNA, Others), Custom Synthesis Services), By Application (Therapeutics Development (Oncology, Infectious Diseases, Others), Biomedical Research), By End-User (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic,…
RNA Extraction and RNA Purification Market: Growth, Trends & Competitive Landsca …
The global RNA Extraction and RNA Purification Market is expected to grow at 6.3% CAGR from 2025 to 2032.
This Market Report is the result of extensive research and analysis conducted by our team of experienced market researchers through -
• 70% efforts of Primary Research
• 15% efforts of Secondary Research
• 15% efforts from the subscription to Paid database providing industry overview, macro and micro economics factors, and financials of private limited…
RNA Targeting Small Molecules Therapeutics Market: Exponential Growth with Risin …
Estimations Predict a CAGR of 29.8% by 2029 in Global RNA Targeting Small Molecules Therapeutics Market Boosted by Precision Medicine, RNA Biomarker Identification and RNA Genetic Manipulation
What Is The Projected Market Size of The Global RNA Targeting Small Molecules Therapeutics Market And Its Growth Rate?
• The market will grow from $6.1 billion in 2024 to $7.87 billion in 2025 at a compound annual growth rate (CAGR) of 28.9%.
• Expected exponential…
Global DNARNA Extraction Kit Market by Type (Cell-free DNA (cfDNA), Sequence-spe …
"DNARNA Extraction Kit Market" is segmented by Company, Region (country), By Type, Application, stakeholders and other participants. This report provides an analysis of revenue and forecast across Type and Application segments for 2023-2032.
The market for DNARNA Extraction Kits has been thoroughly researched via primary and secondary sources to produce this research study. Along with a competitive analysis of the market, segmented by application, type, and geographical trends, it offers a…
Cancer RNA Expression Market to Reap Excessive Revenues by 2028(By sequencing te …
Worldwide cancer is one of the leading cause of death and effective way of treating it still looks unaccomplished in most parts of the world. The factors which influence the successful treatment of cancer are different depending on the stage of diagnosis, treatment availability and availability of trained healthcare professionals coupled with high economic burden of the disease. The gene expression of cancerous cells varies by cancer type and may…