Press release
Graft-Versus-Host Disease Pipeline Assessment 2024 | Clinical Trials, Latest Approvals, Treatment Outlook, Competitive Landscape, Key Companies | Abbisko Therapeutics, Equillium, Theriva Biologics
According to DelveInsight's assessment, globally, approximately 45+ key pharmaceutical companies are developing over 50 drugs in the Graft-Versus-Host Disease (GVHD) therapeutics landscape. These therapies vary in their routes of administration (RoA), mechanisms of action (MoA), and molecular types. Several of these treatments are in advanced stages of clinical development and are anticipated to be launched in the coming years.The Graft versus Host Disease (GvHD) market is anticipated to experience significant growth due to the market penetration of approved therapies, the expected label expansions, and the rapid adoption of emerging therapies. Additionally, increasing awareness of cellular therapies, regulatory support for research, and innovation are also projected to contribute to the market's growth.
Major pharmaceutical giants such as CSL Behring [CSL 964 Alpha-1 antitrypsin (AAT)], Medac [obnitix (MC0518)], Equillium/Biocon [itolizumab], OncoImmune/Merck (MSD) [MK-7110 (CD24Fc)], ElsaLys Biotech/Mediolanum Farmaceutici Spa [Leukotac (inolimomab)], Xenikos [T-Guard], Mesoblast/Osiris Therapeutics [RYONCIL (remestemcel-L; prochymal)], Syndax Pharmaceutical [axatilimab (SNDX-6532)], and Regimmune Corporation (RGI-2001), among others, are actively engaged in the development of novel therapies.
DelveInsight's, "Graft versus host disease (GVHD) Pipeline Insight, 2024 [https://www.delveinsight.com/report-store/graft-versus-host-disease-gvhd-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]," report provides comprehensive insights about 45+ GvHD companies and 50+ pipeline drugs in Graft versus host disease (GVHD) pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
The Graft-Versus-Host Disease Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from pre-clinical developmental to marketed phases. The report also covers a detailed description of the drug, including the mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, collaborations, mergers, acquisition, funding, designations, and other product-related details.
Graft versus host disease Overview
Graft versus host disease (GVHD) is a severe complication that arises when donor immune cells attack the recipient's body following an allogeneic stem cell or bone marrow transplant. This condition occurs because the donor's immune cells identify the recipient's cells as foreign and initiate an immune response against them. GVHD primarily involves T-cells from the donor graft and is categorized into two main types: acute and chronic. Acute GVHD typically develops within the first 100 days post-transplant, presenting symptoms such as skin rashes, jaundice, liver enzyme abnormalities, and diarrhea. Chronic GVHD, on the other hand, emerges after 100 days and can affect multiple organs, leading to symptoms like skin thickening, joint contractures, dry eyes, and lung dysfunction.
The risk of developing GVHD is influenced by several factors, including the degree of HLA matching between donor and recipient, the type of donor, and the recipient's age. A mismatched HLA significantly increases the risk, as does the use of unrelated or haploidentical donors and cord blood transplants compared to matched sibling donors. Older recipients are also more susceptible to GVHD, and the intensity of the conditioning regimen (pre-transplant chemotherapy or radiation) plays a critical role in the disease's development. Diagnosis is based on clinical presentation, biopsy of affected tissues, and the exclusion of other potential causes of symptoms.
Preventive measures are essential in managing GVHD, with prophylactic immunosuppressive therapies such as cyclosporine, tacrolimus, methotrexate, and mycophenolate mofetil commonly used. Treatment of acute GVHD generally involves high-dose corticosteroids, with additional immunosuppressive agents like ruxolitinib and antithymocyte globulin (ATG) used for steroid-refractory cases. Chronic GVHD treatment also includes corticosteroids and calcineurin inhibitors, with newer therapies such as ibrutinib and ruxolitinib for refractory cases. Despite these treatments, GVHD can lead to significant morbidity and mortality due to direct organ damage and increased infection risk from prolonged immunosuppression.
The prognosis for GVHD varies widely; acute GVHD presents an immediate risk of severe outcomes, while chronic GVHD can cause long-term health issues. Effective prevention and treatment strategies are essential to improving patient outcomes and quality of life. Complications of GVHD include chronic infections, secondary cancers, and debilitating organ dysfunction, underscoring the importance of ongoing management and monitoring.
Research is continuously advancing to better understand the mechanisms underlying GVHD and to develop more targeted therapies. Innovations in immunotherapy and personalized medicine approaches hold promise for reducing the incidence and severity of GVHD. These advancements are crucial for enhancing the success rates of transplants and improving the overall prognosis for patients affected by this challenging condition.
Graft-Versus-Host Disease (GVHD) Pipeline Analysis [https://www.delveinsight.com/report-store/graft-versus-host-disease-gvhd-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
The report provides insights into:
*
The report provides detailed insights into the emerging therapies for the treatment of Graft-Versus-Host Disease and the aggregate therapies developed by major pharma companies.
*
It accesses the different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of clinical development.
*
It outlines the key companies involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
The report evaluates the drugs that are under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
It navigates the major collaborations (company-company collaborations and company-academia collaborations), licensing agreements, financing details, data presentation by the pharma giants, and regulatory approval in the Graft-Versus-Host Disease market.
The report is built using data and information traced from the researcher's proprietary databases, company/university websites, clinical trial registries, conferences, SEC filings, investor presentations, and featured press releases from company/university websites and industry-specific third-party sources, etc.
Analysis of Emerging Therapies by Phases
The report covers the emerging products under different phases of clinical development like -
*
Late stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I)
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
Route of Administration
GVHD pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Graft versus host disease products has been categorized under various ROAs, such as
*
Oral
*
Intravenous
*
Subcutaneous
Molecule Type
Graft versus host disease Products have been categorized under various Molecule types, such as
*
Small molecule
*
Cell Therapy
*
Peptides
*
Polymer
*
Small molecule
*
Gene therapy
Learn How the Ongoing Clinical & Commercial Activities will Affect the Graft-Versus-Host Disease Therapeutic Segment @ https://www.delveinsight.com/report-store/graft-versus-host-disease-gvhd-pipeline-insight [https://www.delveinsight.com/report-store/graft-versus-host-disease-gvhd-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Graft-Versus-Host Disease (GVHD) Therapeutics Landscape
Several major pharmaceutical and biotechnology companies are actively engaged in the Graft versus Host Disease (GVHD) therapeutics market. Currently, ElsaLys Biotech is at the forefront of this segment, with its drug candidates in the most advanced stages of clinical development.
Leading Companies in the Graft-Versus-Host Disease (GVHD) Therapeutics Market [https://www.delveinsight.com/sample-request/graft-versus-host-disease-gvhd-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr] Include:
AltruBio, Amgen, ASC Therapeutics, AstraZeneca, Biogen, Bristol-Myers Squibb, Chia Tai Tianqing Pharmaceutical Group, CSL Behring, CTI BioPharma, ElsaLys Biotech (Mediolanum Farmaceutici Spa), Equillium, GlaxoSmithKline, Glia, Incyte Corporation, Jazz Pharmaceuticals, JCR therapeutics, Kadmon Corporation, MaaT Pharma, Medac, Medsenic, Mesoblas, Millennium Pharmaceuticals/Takeda Oncology, Novartis, OncoImmune/Merck (MSD), Osiris Therapeutics, Pfizer, Plexxikon, Prevention Bio, REGiMMUNE, Regimmune Corporation, Roche-Genentech, SCM Lifescience, Syndax Pharmaceuticals, Synthetic Biologics, Takeda, VectivBio, Xenikos, Xenothera, and others.
Graft-Versus-Host Disease (GVHD) Emerging and Marketed Drugs Covered in the Report Include:
*
AbGn-168H (Neihulizumab): AltruBio
*
Arscimed (arsenic trioxide): Medsenic
*
ASC930: ASC Therapeutics
*
Calquence (Acalabrutinib): AstraZeneca
*
CSL 964 Alpha-1 antitrypsin (AAT): CSL Behring
*
Efavaleukin Alfa (AMG 592): Amgen
*
EQ001 (Itolizumab; Bmab600): Equillium/Biocon
*
Itacitinib: Incyte Corporation
*
Jakafi (Ruxolitinib): Incyte Corporation
*
KD025 (Belumosudil): Kadmon Corporation
*
Leukotac (Inolimomab): ElsaLys Biotech (Mediolanum Farmaceutici Spa)
*
MaaT013: MaaT Pharma
*
MK-7110 (CD24Fc): OncoImmune/Merck (MSD)
*
Obnitix (MC0518): Medac
*
Pacritinib (Epjevy; ONX-0803): CTI BioPharma
*
RGI-2001: Regimmune Corporation
*
SNDX-6532 (Axatilimab; GvHD B6352): Syndax Pharmaceutical
*
T-Guard: Xenikos
*
Temcell (Ryoncil; Remestemcel-L; Prochymal): JCR therapeutics/ Mesoblast/ Osiris Therapeutics
*
Teplizumab: Prevention Bio
*
Imbruvica (Ibrutinib): Pharmacyclics (Acquired by Abbvie)/ Janssen
*
Jakafi (Ruxolitinib): Incyte Corporation
*
Ornecia (abatacept): Bristol Mayers Squibb
*
Rezurock (Belumosudil): Kadmon Corporation (Subsidiary of Sanofi)
And Many More
Request the Sample PDF to Get a Better Understanding of the Emerging Drugs and Key Companies @ https://www.delveinsight.com/sample-request/graft-versus-host-disease-gvhd-pipeline-insight [https://www.delveinsight.com/sample-request/graft-versus-host-disease-gvhd-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Contents
1. Report Introduction
2. Executive Summary
3. Graft-Versus-Host Disease Current Treatment Patterns
4. Graft-Versus-Host Disease - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Graft-Versus-Host Disease Late-Stage Products (Phase-III)
7. Graft-Versus-Host Disease Mid-Stage Products (Phase-II)
8. Graft-Versus-Host Disease Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Graft-Versus-Host Disease Discontinued Products
13. Graft-Versus-Host Disease Product Profiles
14. Key Companies in the Graft-Versus-Host Disease Market
15. Key Products in the Graft-Versus-Host Disease Therapeutics Segment
16. Dormant and Discontinued Products
17. Graft-Versus-Host Disease Unmet Needs
18. Graft-Versus-Host Disease Future Perspectives
19. Graft-Versus-Host Disease Analyst Review
20. Appendix
21. Report Methodology
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=graftversushost-disease-pipeline-assessment-2024-clinical-trials-latest-approvals-treatment-outlook-competitive-landscape-key-companies-abbisko-therapeutics-equillium-theriva-biologics]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Graft-Versus-Host Disease Pipeline Assessment 2024 | Clinical Trials, Latest Approvals, Treatment Outlook, Competitive Landscape, Key Companies | Abbisko Therapeutics, Equillium, Theriva Biologics here
News-ID: 3639431 • Views: …
More Releases from ABNewswire
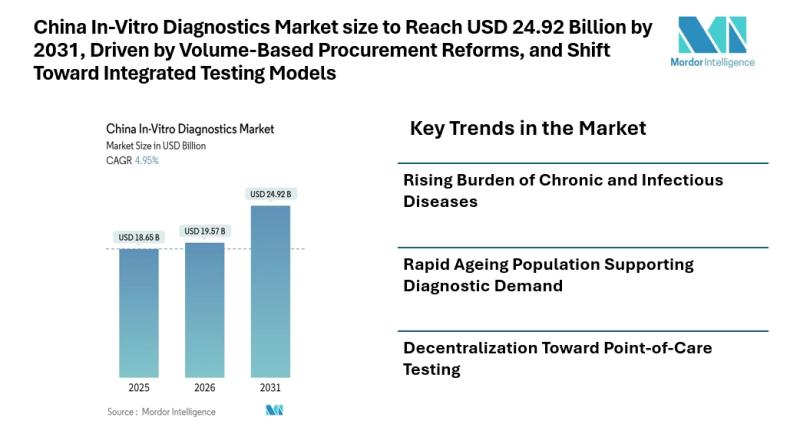
China In-Vitro Diagnostics Market size to Reach USD 24.92 Billion by 2031, Drive …
Mordor Intelligence has published a new report on the china in-vitro diagnostics market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Introduction
According to Mordor Intelligence, the china in-vitro diagnostics market size [https://www.mordorintelligence.com/industry-reports/china-in-vitro-diagnostics-market?utm_source=abnewswire] is projected to reach USD 24.92 billion by 2031, growing from USD 19.57 billion in 2026 at a CAGR of 4.95% during the forecast period. The china in-vitro diagnostics market size reflects steady expansion supported by…

Hyaluronic Acid Market Size to Reach USD 4.07 Billion by 2030 - Mordor Intellige …
Mordor Intelligence has released an in-depth analysis of the hyaluronic acid market, outlining expanding cosmetic, orthopedic, and pharmaceutical applications driving global demand.
Hyaluronic Acid Market Overview
According to Mordor Intelligence, the global hyaluronic acid market size [https://www.mordorintelligence.com/industry-reports/hyaluronic-acid-market?utm_source=abnewswire] reached USD 2.84 billion in 2025 and is projected to grow to USD 4.07 billion by 2030, registering a CAGR of 7.46% during the forecast period.
The strong hyaluronic acid market growth is supported by:
* Increasing…

Scott Bryant Unveils Moon Valley's "Best Value" Listing in Hillcrest East; Signa …
Bryant Real Estate Leverages Data-Driven Performance Metrics to Position New Hillcrest East Property as the Region's Premier Investment Opportunity
PHOENIX, AZ - Scott Bryant, Founder and Team Leader of Bryant Real Estate and a top-performing agent with Keller Williams, has announced the debut of a landmark listing in the Hillcrest East subdivision of Moon Valley. Positioned as "Moon Valley's Best Deal," the property is being introduced at a strategic price point…

Jennifer Rollin Named Best Individual Therapist in Best of Bethesda Awards
Bethesda, MD, USA - Jennifer Rollin, LCSW-C, eating disorder therapist and founder of The Eating Disorder Center, has been named Best Individual Therapist in the 2025 Best of Bethesda Awards. She was selected from among therapists across Montgomery County, Maryland and Upper Northwest Washington, D.C., an honor that reflects both community support and her longstanding commitment to helping individuals recover from eating disorders.
Jennifer Rollin provides eating disorder therapy [https://www.theeatingdisordercenter.com/eatingdisordertherapyrockvilleservices.html] in…
More Releases for Graft
Bone Graft and Graft Substitute Market Latest Research, Top Impacting Factors, G …
The "Bone Graft and Graft Substitute Market Analysis to 2028" is a specialized and in-depth study of the healthcare industry with a special focus on the global market trend analysis. A bone graft is a surgical treatment used to repair problems with bones or joints. Bone grafting, or transplanting of bone tissue, is useful in fixing bones that are impaired from trauma or problem joints. Bone growth around an embedded…
Synthetic Bioactive Bone Graft Substitutes Market Growing need for bone graft su …
Global Synthetic Bioactive Bone Graft Substitutes Market Overview:
The Synthetic Bioactive Bone Graft Substitutes market is a broad category that includes a wide range of products and services related to various industries. This market comprises companies that operate in areas such as consumer goods, technology, healthcare, and finance, among others.
In recent years, the Synthetic Bioactive Bone Graft Substitutes market has experienced significant growth, driven by factors such as increasing consumer demand,…
Bone Graft Substitutes Market Size to Hit $3.8 Billion by 2028 | Bone Graft Subs …
Market Overview:
According to our experience research team, Bone Graft Substitutes Market was valued at USD 2.7 Billion in 2021, and the global Bone Graft Substitutes industry is projected to reach a value of USD 3.8 Billion by 2028, at a CAGR of 5.9% during the forecast period 2022-2028
Vantage Market Research is a collection of market research studies on several industries, such as Chemicals, semiconductors & Electronics, Food & Beverages Technology,…
Graft Delivery Devices Market Facts, Figures and Analytical Insights, 2017 to 20 …
Global Graft Delivery Devices Market: Snapshot
Graft delivery relates to the surgically transplantation of a piece of living tissues and the procedure can be highly essential as well as complex at the same time. Consequently, technological advancements have paved way to graft delivery devices that can minimally breach bone, fat, and vascular parts of the body to transfer drugs during plastic and reconstructive surgeries as well as arthroscopic and orthopedic surgeries.…
Glass Graft Polyols Market 2019| BASF SE, Sinopec, Shell, Oltchim, The Dow Chemi …
Market Research Hub (MRH) has actively included a new research study titled “Global Graft Polyols Market” Research Report 2019 to its wide online repository. The concerned market is discoursed based on a variety of market influential factors such as drivers, opportunities and restraints. This study tends to inform the readers about the current as well as future market scenarios extending up to the period until forecast period limit; 2025. In…
Global Vascular Graft Market Growth Analysis Report 2018-2025Global Vascular Gra …
Research Report 2018-2025 on "Global Vascular Graft Market" which provides an outlook of current market value of Vascular Graft Market as well as the expected forecast of Rate on Investment (ROI) with growing CAGR of XX% in Vascular Graft Market by the end of 2025. The report on the global Vascular Graft market uses the top-down and bottom-up approaches to define, analyze, and describe the Vascular Graft market 2018 trends…